
How is anisole converted into 2-Methoxy toluene?
Answer
585k+ views
Hint: Anisoles have a methoxy group on the benzene ring, it is an ortho substituting group. When methyl group attacks at the anisole group, it gets attached to the ortho position. $AlC{{l}_{3}}$ act as a lewis acid.
Complete step by step answer:
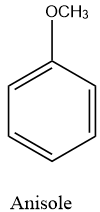
Anisole or methoxybenzene, is an organic compound with the formula $C{{H}_{3}}O{{C}_{6}}{{H}_{5}}$. It is a colourless liquid with a smell reminiscent of anise seed, and in many of its derivatives are found in natural and artificial fragrances. The structure of anisole is as follows:
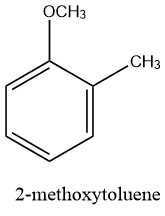
The structure of 2-methoxytoluene is given as follows:
The following synthesis can be done using Friedel Craft alkylation. The friedel-crafts reaction are a set of reactions developed by Charles Friedel and James Craft in 1877 to attach substituents to an aromatic ring. Friedel-Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.
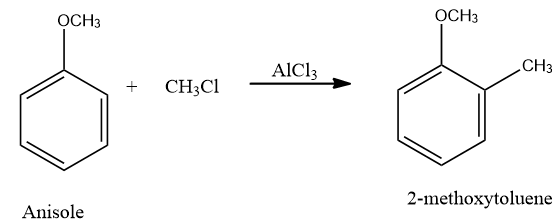
Friedel Craft reaction proceeds through use of a lewis acid. In the question given above, we have anisole as a substrate and $AlC{{l}_{3}}$ is used as a lewis acid.Methyl chloride is the attacking species. Lewis acid, $AlC{{l}_{3}}$, takes up the chloride from the methyl chloride and electrophile $C{{H}_{3}}^{+}$, methyl cation is generated. This methyl cation attacks on the anisole, at the ortho position.
The reaction of the conversion of anisole to 2-methoxytoluene is as follows:
Note: $AlC{{l}_{3}}$ is a lewis acid because it can accept electrons. Aluminium has vacant orbitals and is strong electrophile due to presence of high electron negative Chlorine atoms which reduces the electron density from the central atom.
Complete step by step answer:
Anisole or methoxybenzene, is an organic compound with the formula $C{{H}_{3}}O{{C}_{6}}{{H}_{5}}$. It is a colourless liquid with a smell reminiscent of anise seed, and in many of its derivatives are found in natural and artificial fragrances. The structure of anisole is as follows:

The structure of 2-methoxytoluene is given as follows:

The following synthesis can be done using Friedel Craft alkylation. The friedel-crafts reaction are a set of reactions developed by Charles Friedel and James Craft in 1877 to attach substituents to an aromatic ring. Friedel-Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.
Friedel Craft reaction proceeds through use of a lewis acid. In the question given above, we have anisole as a substrate and $AlC{{l}_{3}}$ is used as a lewis acid.Methyl chloride is the attacking species. Lewis acid, $AlC{{l}_{3}}$, takes up the chloride from the methyl chloride and electrophile $C{{H}_{3}}^{+}$, methyl cation is generated. This methyl cation attacks on the anisole, at the ortho position.
The reaction of the conversion of anisole to 2-methoxytoluene is as follows:

Note: $AlC{{l}_{3}}$ is a lewis acid because it can accept electrons. Aluminium has vacant orbitals and is strong electrophile due to presence of high electron negative Chlorine atoms which reduces the electron density from the central atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




