
How are ethylamine and ethyl methyl amine distinguished by using nitrous acid?
Answer
571.5k+ views
Hint: The different amines react differently with nitrous acid. Primary amines form alcohol. Secondary amines form nitroso compounds. The tertiary amine does not react. We can distinguish between both the compounds with the help of two reactions in presence of nitrous acid.
Complete step by step solution:
Ethylamine is a primary amine and ethyl methyl amine is a secondary amine. Both react differently with nitrous acid. So, this reaction is used for distinguished primary, secondary and tertiary amine compounds.
The product of the reaction of ethylamine with nitrous acid is shown as follows:
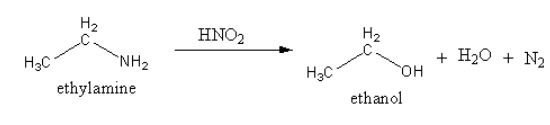
The reaction of ethylamine with nitrous acid gives ethanol, water and nitrogen gas. Nitrogen is colourless and odourless gas. By the evolution of the gas, primary amines are identified.
The product of reaction of ethyl methyl amine with nitrous acid is shown as follows:
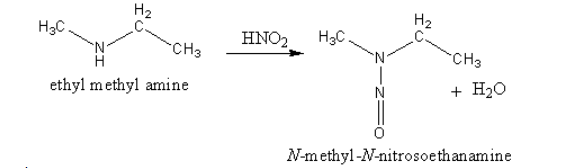
The reaction of ethyl methyl amine with nitrous acid gives N-methyl-N-nitroso ethylamine and water. The N-methyl-N-nitroso ethanamine is yellow coloured oil. So, secondary amines are identified by the formation of a yellow oil.
Therefore, ethylamine forms ethanol and ethyl methyl amine forms nitrosamine with nitrous acid. So, both ethylamine and ethyl methyl amine can be distinguished by using nitrous acid as during the reaction of ethylamine, the evolution of the nitrogen gas observed and during the reaction of ethyl methyl amine, the yellow oily product forms.
Note: The tertiary amines do not react with nitrous acid. The amines are basic so, tertiary amine gets protonated in presence of nitrous acid and form cation of a tertiary amine. Thus by the reaction of an amine with nitrous acid, it can be identified that the amine is primary, secondary or tertiary. The nitrous acid is also used for the formation of diazonium salt of amines.
Complete step by step solution:
Ethylamine is a primary amine and ethyl methyl amine is a secondary amine. Both react differently with nitrous acid. So, this reaction is used for distinguished primary, secondary and tertiary amine compounds.
The product of the reaction of ethylamine with nitrous acid is shown as follows:

The reaction of ethylamine with nitrous acid gives ethanol, water and nitrogen gas. Nitrogen is colourless and odourless gas. By the evolution of the gas, primary amines are identified.
The product of reaction of ethyl methyl amine with nitrous acid is shown as follows:

The reaction of ethyl methyl amine with nitrous acid gives N-methyl-N-nitroso ethylamine and water. The N-methyl-N-nitroso ethanamine is yellow coloured oil. So, secondary amines are identified by the formation of a yellow oil.
Therefore, ethylamine forms ethanol and ethyl methyl amine forms nitrosamine with nitrous acid. So, both ethylamine and ethyl methyl amine can be distinguished by using nitrous acid as during the reaction of ethylamine, the evolution of the nitrogen gas observed and during the reaction of ethyl methyl amine, the yellow oily product forms.
Note: The tertiary amines do not react with nitrous acid. The amines are basic so, tertiary amine gets protonated in presence of nitrous acid and form cation of a tertiary amine. Thus by the reaction of an amine with nitrous acid, it can be identified that the amine is primary, secondary or tertiary. The nitrous acid is also used for the formation of diazonium salt of amines.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




