
What are meta, ortho, para groups in a benzene ring? Show with the help of a diagram.
Answer
501k+ views
Hint: Benzene is an aromatic hydrocarbon having chemical formula $ {C_6}{H_6} $ . By replacing one or more than one hydrogen atom in the benzene ring with the functional group, the substituted benzene derivatives can be obtained. When there exists more than one substituent, the positions can be named as ortho- (o), meta- (m), para- (p).
Complete answer:
For naming the substituted benzene compounds, the prefix ortho, para and meta are used according to the relative positions of the functional groups. In substituted benzene derivatives, the ortho position refers to the two substituent groups which occupy positions adjacent to each other and it may also be referred to as (1,2) position in the diagram. The diagram representing the ortho position in the benzene ring is shown as follows:
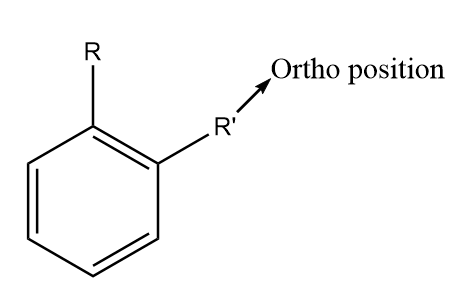
The meta position in substituted benzene derivatives refers to the substituent groups which occupy positions 1 and 3 corresponding to R. The diagram representing the meta position in the benzene ring is shown as follows:
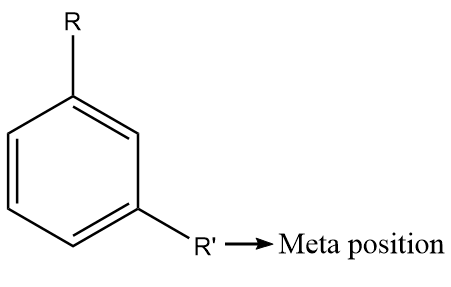
The para positions refer to the substituents occupying the opposite ends i.e., positions 1 and 4 corresponding to R. The diagram representing the para position in the benzene ring is shown as follows:
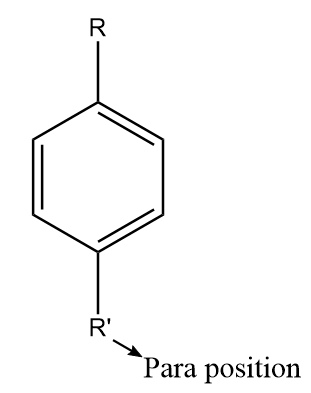
Note:
It is important to note that while naming the substituted benzene derivatives, the two substituted functional groups cannot have the same name and same priority. The benzene ring which has substituents more than six carbons, the benzene ring is noted with phenyl prefix whereas the substituents which contains less than six carbons, the alkyl chain is added as prefix ending with -yl.
Complete answer:
For naming the substituted benzene compounds, the prefix ortho, para and meta are used according to the relative positions of the functional groups. In substituted benzene derivatives, the ortho position refers to the two substituent groups which occupy positions adjacent to each other and it may also be referred to as (1,2) position in the diagram. The diagram representing the ortho position in the benzene ring is shown as follows:

The meta position in substituted benzene derivatives refers to the substituent groups which occupy positions 1 and 3 corresponding to R. The diagram representing the meta position in the benzene ring is shown as follows:

The para positions refer to the substituents occupying the opposite ends i.e., positions 1 and 4 corresponding to R. The diagram representing the para position in the benzene ring is shown as follows:

Note:
It is important to note that while naming the substituted benzene derivatives, the two substituted functional groups cannot have the same name and same priority. The benzene ring which has substituents more than six carbons, the benzene ring is noted with phenyl prefix whereas the substituents which contains less than six carbons, the alkyl chain is added as prefix ending with -yl.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




