
What are sawhorse projections in organic chemistry ?
Answer
519.6k+ views
Hint : A sawhorse projection unlike the molecular formula and other molecular representations views the molecule down in a particular angle around a carbon-carbon bond. It has both staggered and eclipsed conformation like the Newman projection.
Complete Step By Step Answer:
Given the hint, a sawhorse projection is a representation of a molecule when viewed from a particular angle around a carbon-carbon bond. Unlike the Newman projection, the adjacent carbon atoms are not crushed into a single center, instead, the C-C bond is drawn and the other three groups attached to the carbon atom are separated by $ {120^ \circ } $ . It can be better explained by an example.
Take the case of n-butane $ C{H_3}C{H_2}C{H_2}C{H_3} $ ,
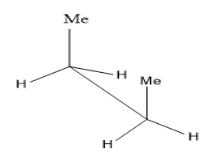
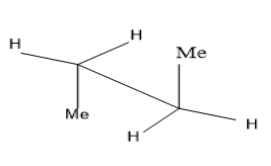
The first image is the eclipsed conformation of butane while the second one is its staggered conformation (anti) . Two carbon atoms are joined by a solid line and the other three functional groups attached to it have $ {120^ \circ } $ separation. Anti and gauche conformations are the different staggered conformations. Rotations around the C-C single bond gives rise to conformers. Among these, eclipsed conformation seems to be least stable due to torsional strain between two methyl groups and the anti ( staggered) more stable due to large separation between the two methyl groups.
Note :
A sawhorse projection helps in a better visualization of conformation of a molecule. It clearly depicts the three dimensional geometry and interaction between the groups of adjacent carbon atoms. This can help us to identify the characteristic properties of the molecules easier like its mirror image, stereo selectivity, reaction mechanism etc.
Complete Step By Step Answer:
Given the hint, a sawhorse projection is a representation of a molecule when viewed from a particular angle around a carbon-carbon bond. Unlike the Newman projection, the adjacent carbon atoms are not crushed into a single center, instead, the C-C bond is drawn and the other three groups attached to the carbon atom are separated by $ {120^ \circ } $ . It can be better explained by an example.
Take the case of n-butane $ C{H_3}C{H_2}C{H_2}C{H_3} $ ,


The first image is the eclipsed conformation of butane while the second one is its staggered conformation (anti) . Two carbon atoms are joined by a solid line and the other three functional groups attached to it have $ {120^ \circ } $ separation. Anti and gauche conformations are the different staggered conformations. Rotations around the C-C single bond gives rise to conformers. Among these, eclipsed conformation seems to be least stable due to torsional strain between two methyl groups and the anti ( staggered) more stable due to large separation between the two methyl groups.
Note :
A sawhorse projection helps in a better visualization of conformation of a molecule. It clearly depicts the three dimensional geometry and interaction between the groups of adjacent carbon atoms. This can help us to identify the characteristic properties of the molecules easier like its mirror image, stereo selectivity, reaction mechanism etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




