
What are the different kinds of f orbitals?
Answer
504.3k+ views
Hint: Every atom or element in the periodic table contains electrons. All the electrons are going to enter into the fixed orbitals and the electrons continuously move in the stationary orbits. The atomic orbitals are four types and they are s, p d, and f.
Complete answer:
- In the question it is asked to write the different kinds of f-orbitals.
- There are four blocks in the periodic table and they are s, p, d and f blocks.
- The elements are classified into different kinds based on the position of the valence electrons.
- The f-block is positioned at the bottom of the periodic table.
- The f block is going to start with a lanthanum element.
- For the f block elements there 7 f orbitals are present.
- For s orbital there is only an orbital, for p orbital there are three sub-orbitals, for d-orbital there are five sub-orbitals and for f orbital there are 7 sub-orbitals.
- Means f orbital has a capability to accommodate 14 electrons in its orbital.
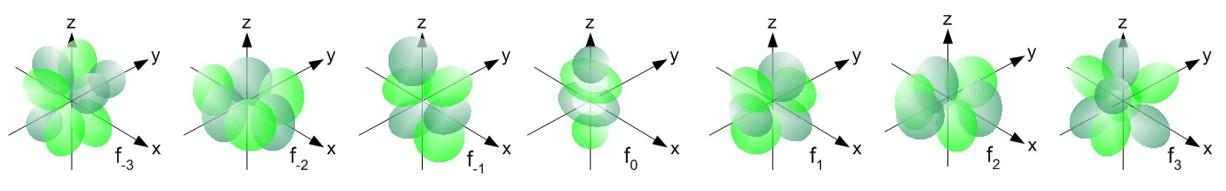
- The shapes of the f orbitals are as follows, we can see the different shapes of the seven sub-orbitals of the f.
Note:
All atoms in the periodic table do not contain f-orbitals. The elements which are present in the f block only contain f orbitals and they can accommodate fourteen electrons in their sub-orbitals. The f orbitals are very complex.
Complete answer:
- In the question it is asked to write the different kinds of f-orbitals.
- There are four blocks in the periodic table and they are s, p, d and f blocks.
- The elements are classified into different kinds based on the position of the valence electrons.
- The f-block is positioned at the bottom of the periodic table.
- The f block is going to start with a lanthanum element.
- For the f block elements there 7 f orbitals are present.
- For s orbital there is only an orbital, for p orbital there are three sub-orbitals, for d-orbital there are five sub-orbitals and for f orbital there are 7 sub-orbitals.
- Means f orbital has a capability to accommodate 14 electrons in its orbital.
- The shapes of the f orbitals are as follows, we can see the different shapes of the seven sub-orbitals of the f.

Note:
All atoms in the periodic table do not contain f-orbitals. The elements which are present in the f block only contain f orbitals and they can accommodate fourteen electrons in their sub-orbitals. The f orbitals are very complex.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




