
How are the following compounds prepared?
i.Benzyl alcohol from benzyl chloride
ii.Propane-1-ol from propanal.
Answer
579.9k+ views
Hint: Alcohol is defined as the hydroxy derivative of aliphatic hydrocarbons. In aliphatic hydrocarbons, there is a replacement of hydrogen atoms with an equal number of hydroxyl groups which results in the formation of alcohol.
Complete step by step answer:
a.Benzyl alcohol is prepared by hydrolysis of benzyl chloride. During the reaction, benzyl chloride is heated with aqueous potassium hydroxide or sodium hydroxide. The chemical reaction for the preparation of benzyl alcohol from benzyl chloride is given as:
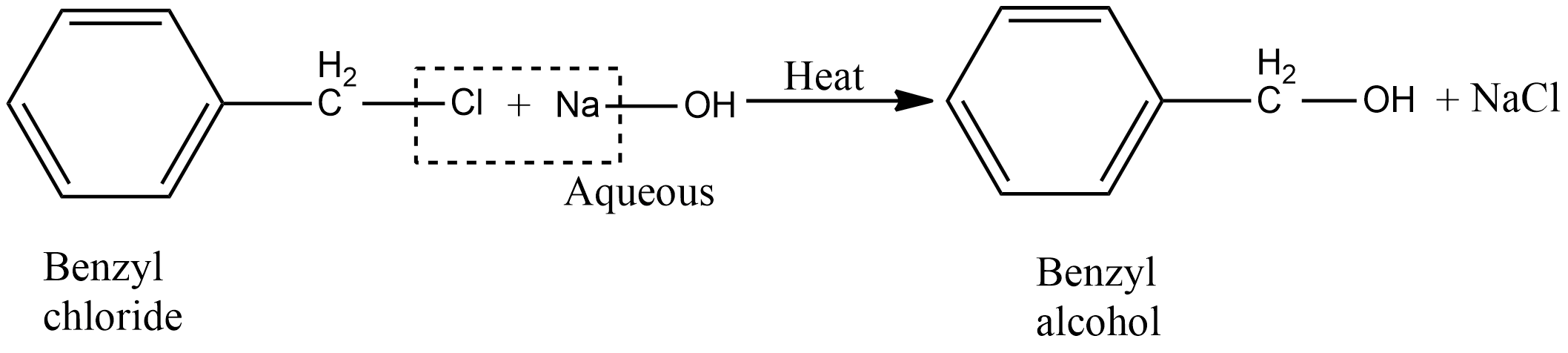
During this reaction instead of aqueous sodium hydroxide or potassium hydroxide, we can use moist silver oxide also.
b.Since propanal contains aldehyde as a functional group. Aldehyde is reduced to corresponding alcohol by the addition of hydrogen in the presence of catalysts such as finely divided platinum, palladium, or ruthenium. Hence we can prepare propan-1-ol by catalytic reduction of propanal in the presence of finely divided platinum, palladium, or ruthenium as:
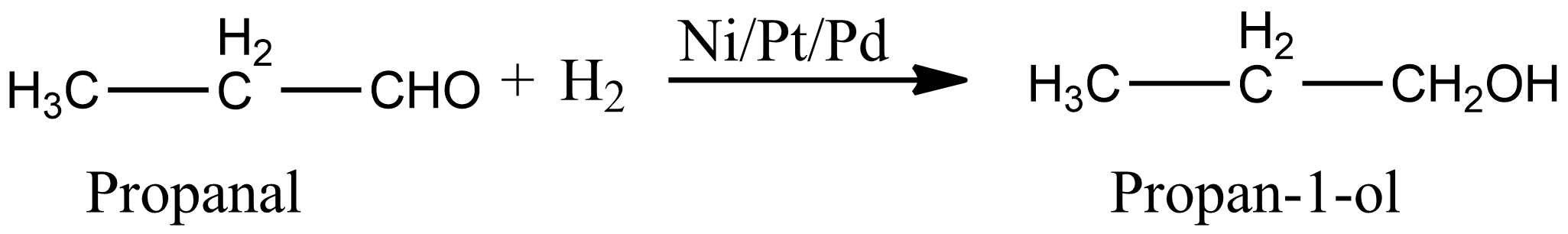
There are some other reducing agents which are used for the reduction of an aldehyde into alcohol. For example, aldehyde can be reduced to alcohol by treating it with sodium in the presence of ethanol, and also we can use complex metal hydrides such as lithium aluminum hydride or sodium borohydride.
Note:
Many alcohols find wide application in industries as well as in our daily life. For example, an ordinary spirit is used as an antiseptic. Ethanol is used for polishing the furniture. The sugar (sucrose) we used in our daily life for eating purposes. Cotton (cellulose) we used for making fibers. Hexachlorophene, which is a phenolic compound, is a constituent of several types of mouthwash, medical skin cleanser.
Complete step by step answer:
a.Benzyl alcohol is prepared by hydrolysis of benzyl chloride. During the reaction, benzyl chloride is heated with aqueous potassium hydroxide or sodium hydroxide. The chemical reaction for the preparation of benzyl alcohol from benzyl chloride is given as:

During this reaction instead of aqueous sodium hydroxide or potassium hydroxide, we can use moist silver oxide also.
b.Since propanal contains aldehyde as a functional group. Aldehyde is reduced to corresponding alcohol by the addition of hydrogen in the presence of catalysts such as finely divided platinum, palladium, or ruthenium. Hence we can prepare propan-1-ol by catalytic reduction of propanal in the presence of finely divided platinum, palladium, or ruthenium as:
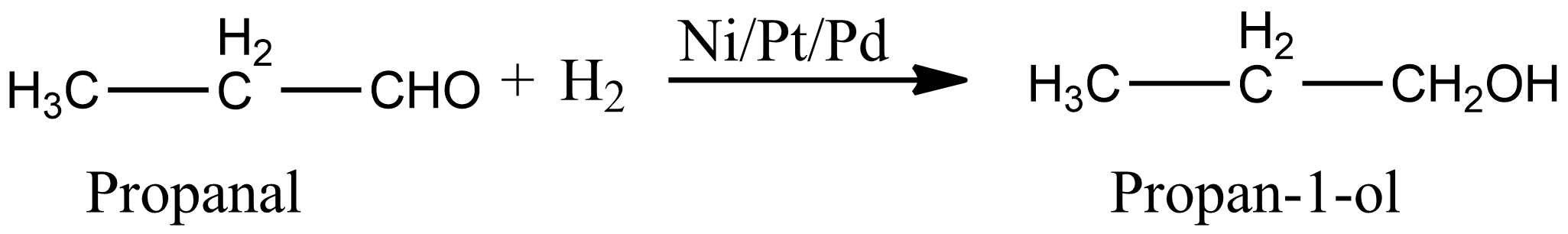
There are some other reducing agents which are used for the reduction of an aldehyde into alcohol. For example, aldehyde can be reduced to alcohol by treating it with sodium in the presence of ethanol, and also we can use complex metal hydrides such as lithium aluminum hydride or sodium borohydride.
Note:
Many alcohols find wide application in industries as well as in our daily life. For example, an ordinary spirit is used as an antiseptic. Ethanol is used for polishing the furniture. The sugar (sucrose) we used in our daily life for eating purposes. Cotton (cellulose) we used for making fibers. Hexachlorophene, which is a phenolic compound, is a constituent of several types of mouthwash, medical skin cleanser.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




