
What are the oxidation number of $2C{l^ - }$ and $C{l_2}$
Answer
525.6k+ views
Hint :Oxidation number is the number of electrons gained or lost during the oxidation. If the given sign is positive it indicates electron loss. If the sign is negative it indicates electron gain.
Complete Step By Step Answer:
For $2C{l^ - }$ iron it indicates that there are $2$ions of $C{l^ - }$present. That means the oxidation number is $2 \times ( - 1) = - 2$
Whereas for $C{l_2}$ it indicates $2$atoms of $Cl$ present. These to atoms share bonds to form chlorine gas.
There are total of $7$ valance electrons per chlorine atom making it a total of $14$in $C{l_2}$
These electron share a bond between $2$chlorine atom to form $C{l_2}$.
One atom receives a charge of $ - 1$and the other receives a charge of $ + 1$ making the total of $ - 1( + 1) = 0$
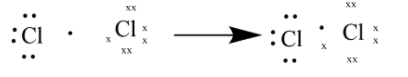
As seen in the above diagram each $Cl$ atom shares one of the valance electrons to form a bond.
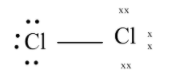
The final structure looks like
Therefore the oxidation number of $C{l_2}$is $0$.
Note :
Chlorine gas is used in disinfection, paper producing industries etc. an atom having the highest electronegativity is given negative oxidation number.
Complete Step By Step Answer:
For $2C{l^ - }$ iron it indicates that there are $2$ions of $C{l^ - }$present. That means the oxidation number is $2 \times ( - 1) = - 2$
Whereas for $C{l_2}$ it indicates $2$atoms of $Cl$ present. These to atoms share bonds to form chlorine gas.
There are total of $7$ valance electrons per chlorine atom making it a total of $14$in $C{l_2}$
These electron share a bond between $2$chlorine atom to form $C{l_2}$.
One atom receives a charge of $ - 1$and the other receives a charge of $ + 1$ making the total of $ - 1( + 1) = 0$

As seen in the above diagram each $Cl$ atom shares one of the valance electrons to form a bond.
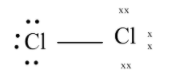
The final structure looks like
Therefore the oxidation number of $C{l_2}$is $0$.
Note :
Chlorine gas is used in disinfection, paper producing industries etc. an atom having the highest electronegativity is given negative oxidation number.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




