
What are the reaction sequences of glucosazone formation?
Answer
598.5k+ views
Hint – You can start by describing glucose and its uses. Then move on to describe phenylhydrazine and its uses. Then describe the reaction between glucose and phenylhydrazine. Then finally describe glucosazone and its properties.
Complete step by step solution:
Glucose is a six carbon compound made by plants during the process of photosynthesis. During photosynthesis plants use carbon dioxide and water and with the help of sunlight in a complicated process produce glucose. Glucose is a monosaccharide that is found in abundance in nature. Glucose can be found anywhere from the sweetener used in your food to the cellulose in the plant cell wall.
Phenylhydrazine is a chemical compound having the molecular formula
 Phenylhydrazine is in an oily form at room temperature. When it comes in contact with air, their color changes from yellow color to dark red color. Phenylhydrazine is produced by the following reactions. The first reaction is between sodium nitrite with aniline in the presence of hydrogen chloride. The former reaction produces diazonium salt which is further reduced using sodium sulfite with sodium hydroxide and thus phenylhydrazine is formed. Phenylhydrazine under Fischer indole synthesis produces indoles.
Phenylhydrazine is in an oily form at room temperature. When it comes in contact with air, their color changes from yellow color to dark red color. Phenylhydrazine is produced by the following reactions. The first reaction is between sodium nitrite with aniline in the presence of hydrogen chloride. The former reaction produces diazonium salt which is further reduced using sodium sulfite with sodium hydroxide and thus phenylhydrazine is formed. Phenylhydrazine under Fischer indole synthesis produces indoles.
The reaction of glucosazone formation are –
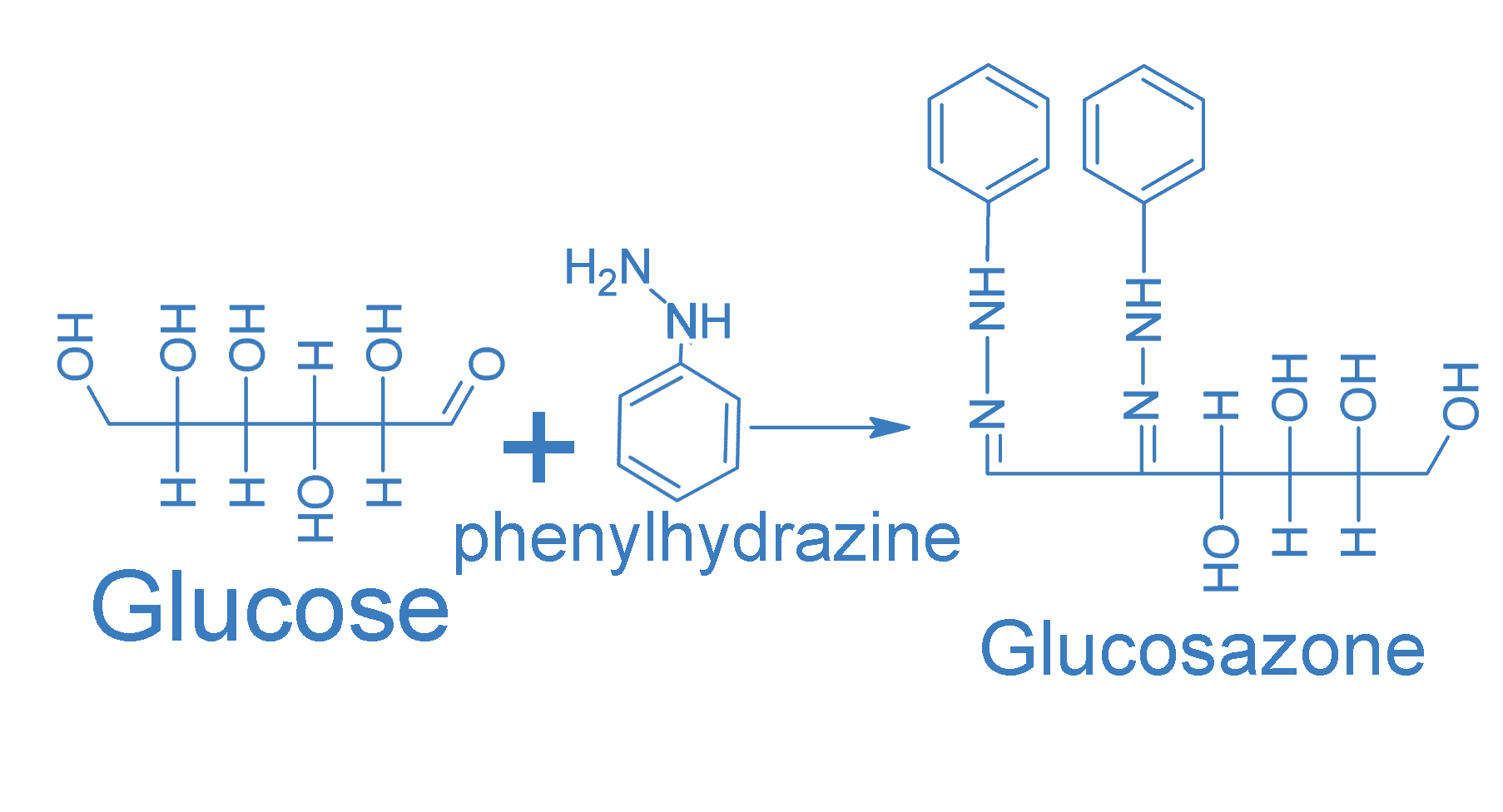
In this reaction one molecule of glucose reacts with two molecules of phenyl hydrazine to create glucosazone.
Glucosazone is a yellow crystalline substance that is often used to identify the presence of glucose. It's chemically very similar to fructosazone and mannosazone
Note - Glucosazone is an example of osazone.Osazones are a class of carbohydrates formed by the reaction of reducing sugars with phenylhydrazine. Ozone are colored compounds with crystalline in nature (remember than glucosazone is a yellow crystalline substance). Fructosazone and mannosazone are some more examples of osazone.
Complete step by step solution:
Glucose is a six carbon compound made by plants during the process of photosynthesis. During photosynthesis plants use carbon dioxide and water and with the help of sunlight in a complicated process produce glucose. Glucose is a monosaccharide that is found in abundance in nature. Glucose can be found anywhere from the sweetener used in your food to the cellulose in the plant cell wall.
Phenylhydrazine is a chemical compound having the molecular formula

The reaction of glucosazone formation are –

In this reaction one molecule of glucose reacts with two molecules of phenyl hydrazine to create glucosazone.
Glucosazone is a yellow crystalline substance that is often used to identify the presence of glucose. It's chemically very similar to fructosazone and mannosazone
Note - Glucosazone is an example of osazone.Osazones are a class of carbohydrates formed by the reaction of reducing sugars with phenylhydrazine. Ozone are colored compounds with crystalline in nature (remember than glucosazone is a yellow crystalline substance). Fructosazone and mannosazone are some more examples of osazone.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




