
What are the shapes of the molecules of water and boron trifluoride?
${{H}_{2}}O$ $B{{F}_{3}}$ A Linear Pyramidal B Linear Trigonal C Non-linear Pyramidal D Non-linear trigonal
(a) A
(b) B
(c) C
(d) D
| ${{H}_{2}}O$ | $B{{F}_{3}}$ | |
| A | Linear | Pyramidal |
| B | Linear | Trigonal |
| C | Non-linear | Pyramidal |
| D | Non-linear | trigonal |
Answer
575.7k+ views
Hint: By calculating the by hybridization of ${{H}_{2}}O$ and $B{{F}_{3}}$ (it is process of inter-mixing of the orbitals to form new orbitals of equivalent energy),we can know about their shapes. The hybridization can be calculated by the formula:
H= $\dfrac{1}{2}$ (V+M-C+A)
Here, V represents the number of electrons in the valence shell of the atom, M represents the monovalent atoms attached to that atom and C and A represents the charges of the cations and anions. Now, Identify the correct statement.
Complete answer:
First of all, let’s discuss what hybridization is. By the term hybridization we mean the phenomenon of inter-mixing of the orbitals of slightly different energies so as to redistribute their energies and to give a new set of orbitals of equivalent energies and shape.
First, we have to find the hybridization of $\text{P}{{\text{F}}_{5}}$ molecule by the formula as: H= $\dfrac{1}{2}$ (V+M-C+A) -(1)
Here, V= number of the valence electrons in the atom, M= number of the monovalent atoms bonded to the central atom, C=the charge on the cation and A= the charge on the anion.
Now, calculating the hybridization of both ${{H}_{2}}O$ and $B{{F}_{3}}$by using this formula as;
In ${{H}_{2}}O$ ;
Number of valence electrons in oxygen =6
Number of monovalent electrons=2
Then, we get;
Hybridization of ${{H}_{2}}O$=$\dfrac{1}{2}(6+2)=\dfrac{1}{2}\times 8=4$
Since, its hybridization is $s{{p}^{3}}$, therefore, it has pyramidal geometry due to the presence of two lone pairs of electrons and two bond pairs instead of the tetrahedral geometry and thus, has non-linear shape as;

Similarly, in $B{{F}_{3}}$;
Number of valence electrons in B=3
Number of monovalent atoms=3
Then, we get;
Hybridization of $B{{F}_{3}}$ =$\dfrac{1}{2}(3+3)=\dfrac{1}{2}\times 6=3$ =3
Since, its hybridization is $s{{p}^{2}}$, therefore, it has trigonal planar geometry due to the presence of three bond pairs as;
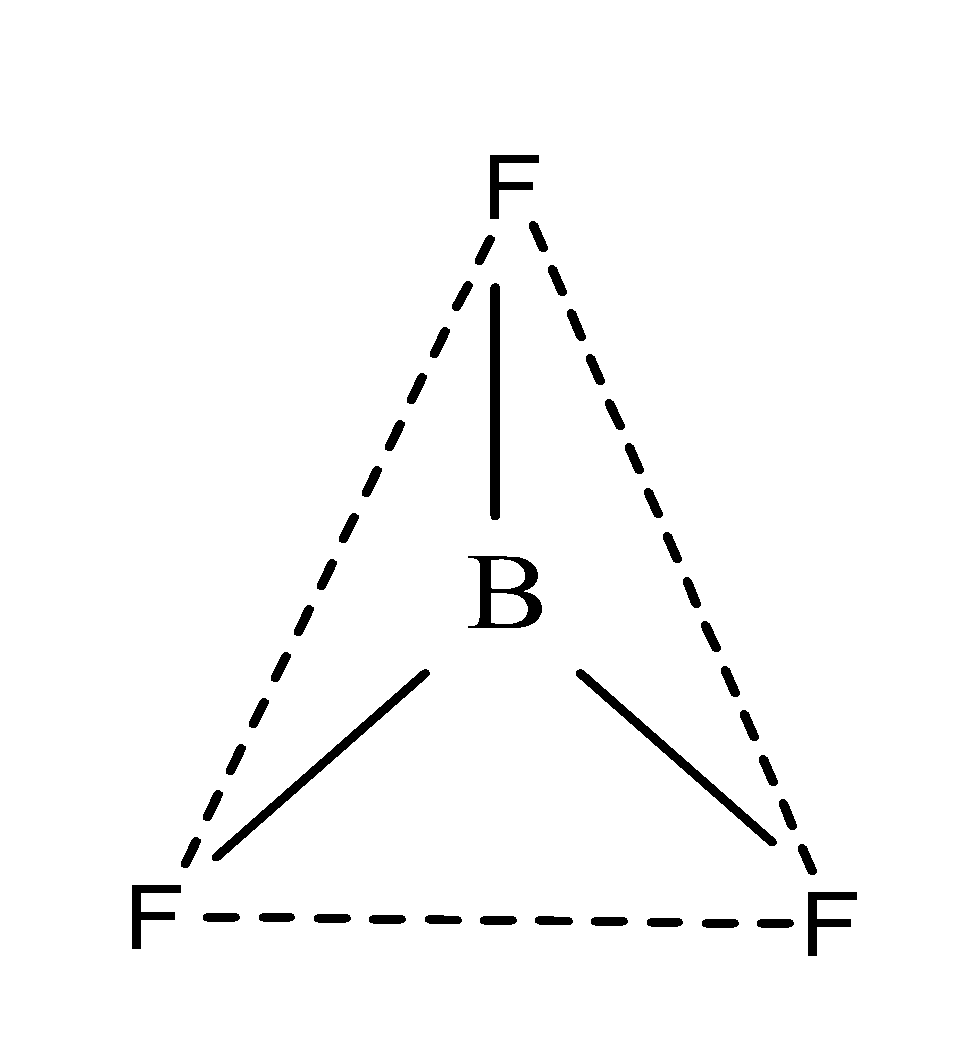
So, the shapes of the molecules of water and boron trifluoride are non- linear and trigonal respectively.
Hence, option(d) is correct.
Note: Hybridization helps us to predict the shape, geometry and structure of the molecules and isostructural are those structures which have the same number of atoms and they are arranged in the same way in the similar structures.
H= $\dfrac{1}{2}$ (V+M-C+A)
Here, V represents the number of electrons in the valence shell of the atom, M represents the monovalent atoms attached to that atom and C and A represents the charges of the cations and anions. Now, Identify the correct statement.
Complete answer:
First of all, let’s discuss what hybridization is. By the term hybridization we mean the phenomenon of inter-mixing of the orbitals of slightly different energies so as to redistribute their energies and to give a new set of orbitals of equivalent energies and shape.
First, we have to find the hybridization of $\text{P}{{\text{F}}_{5}}$ molecule by the formula as: H= $\dfrac{1}{2}$ (V+M-C+A) -(1)
Here, V= number of the valence electrons in the atom, M= number of the monovalent atoms bonded to the central atom, C=the charge on the cation and A= the charge on the anion.
Now, calculating the hybridization of both ${{H}_{2}}O$ and $B{{F}_{3}}$by using this formula as;
In ${{H}_{2}}O$ ;
Number of valence electrons in oxygen =6
Number of monovalent electrons=2
Then, we get;
Hybridization of ${{H}_{2}}O$=$\dfrac{1}{2}(6+2)=\dfrac{1}{2}\times 8=4$
Since, its hybridization is $s{{p}^{3}}$, therefore, it has pyramidal geometry due to the presence of two lone pairs of electrons and two bond pairs instead of the tetrahedral geometry and thus, has non-linear shape as;

Similarly, in $B{{F}_{3}}$;
Number of valence electrons in B=3
Number of monovalent atoms=3
Then, we get;
Hybridization of $B{{F}_{3}}$ =$\dfrac{1}{2}(3+3)=\dfrac{1}{2}\times 6=3$ =3
Since, its hybridization is $s{{p}^{2}}$, therefore, it has trigonal planar geometry due to the presence of three bond pairs as;

So, the shapes of the molecules of water and boron trifluoride are non- linear and trigonal respectively.
Hence, option(d) is correct.
Note: Hybridization helps us to predict the shape, geometry and structure of the molecules and isostructural are those structures which have the same number of atoms and they are arranged in the same way in the similar structures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




