
What are two conformations of cis-1,4-dimethyl cyclohexane?
Answer
524.7k+ views
Hint : Just two stereoisomers, cis- and trans- 1,4-dimethyl cyclohexane, have an internal symmetry plane. As a result, both are meso compounds (optically inactive). Only cis-1,3-dimethyl cyclohexane, unlike 1,4-dimethyl cyclohexane, has an internal symmetry plane, whereas trans-1,3-dimethyl cyclohexane does not.
Complete Step By Step Answer:
Cis-1,4-dimethyl cyclohexane has one chair conformation and two boat conformations. The chair conformation of cyclohexane is more stable than the boat conformation since the $C - H$ bonds are similarly axial and equatorial in the chair conformation, i.e., six of the twelve $C - H$bonds are axial and six are equatorial, and each carbon has one axial and one equatorial $C - H$bond.
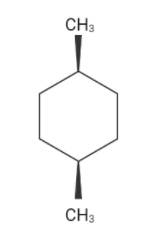
The chain structure of cis-1,4-dimethyl cyclohexane
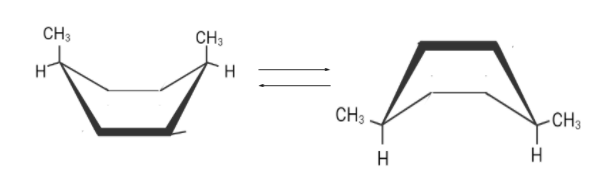
There are two flipped boat conformations . Although both are different but the right one is more stable.
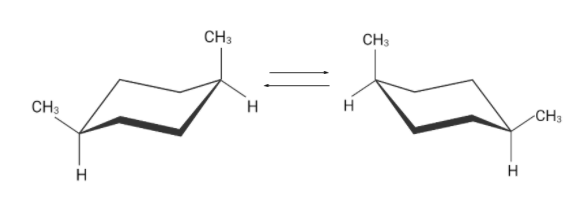
Similarly there are two flipped cyclohexane chairs. These two conformations are identical.
Note :
This compound can be used in various real life applications. The absorption of ultrasonic waves that can be calculated using a reverberation method has been studied using cis-1,4-dimethylcyclohexane.
Complete Step By Step Answer:
Cis-1,4-dimethyl cyclohexane has one chair conformation and two boat conformations. The chair conformation of cyclohexane is more stable than the boat conformation since the $C - H$ bonds are similarly axial and equatorial in the chair conformation, i.e., six of the twelve $C - H$bonds are axial and six are equatorial, and each carbon has one axial and one equatorial $C - H$bond.
The chain structure of cis-1,4-dimethyl cyclohexane

There are two flipped boat conformations . Although both are different but the right one is more stable.

Similarly there are two flipped cyclohexane chairs. These two conformations are identical.

Note :
This compound can be used in various real life applications. The absorption of ultrasonic waves that can be calculated using a reverberation method has been studied using cis-1,4-dimethylcyclohexane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




