
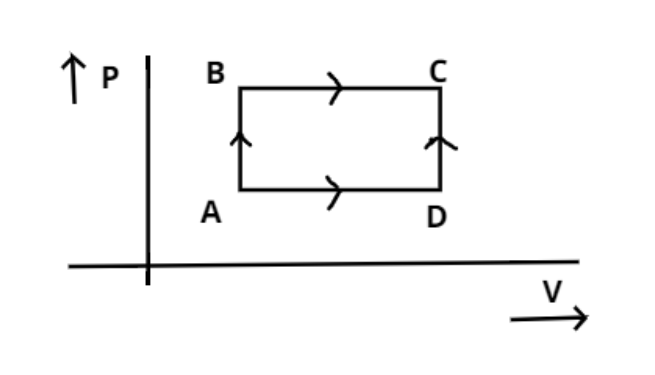
As shown in the figure the amount of heat absorbed along that path ABC is 90 J and the amount of work done by the system is 30 J. If the amount of work done along the path ADC is 20 J. Find the amount of heat absorbed.

Answer
598.8k+ views
Hint – In this question let the amount of heat absorbed along the path ADC be (${Q_2}$). Then use the first law of thermodynamics that change in internal energy for a closed system is always constant. And is given as ($\Delta U = Q - W$). Suppose the change in internal energy along path ABC be ($\Delta {U_1}$) and along the path ADC be ($\Delta {U_2}$). Now this change in internal energy along the paths ADC and ABC will be the same. This will help to approach the problem.
Step-By-Step answer:
Given data:
Heat absorbed (${Q_1}$) along the path ABC is 90 J.
$ \Rightarrow {Q_1} = 90J$
And the amount of work done (${W_1}$) by the system along the path ABC is 30 J.
$ \Rightarrow {W_1} = 30J$
And the amount of work done (${W_2}$) by the system along the path ADC is 20 J.
$ \Rightarrow {W_2} = 20J$
Let the amount of heat absorbed along the path ADC be (${Q_2}$)
Now according to the first law of thermodynamics change in internal energy is always constant and it is equal to the difference of heat absorbed along the path and the amount of work done along the same path.
$ \Rightarrow \Delta U$= Q – W
Where, $\Delta U$ = change in internal energy, Q = heat added or absorbed and W = work done.
Therefore change in internal energy along the path ABC is equal to the change in internal energy along the path ADC.
Let change in internal energy along the path ABC = $\Delta {U_1} = {Q_1} - {W_1}$
And change in internal energy along the path ADC = $\Delta {U_2} = {Q_2} - {W_2}$
Therefore, $\Delta {U_1} = \Delta {U_2}$
$ \Rightarrow {Q_1} - {W_1} = {Q_2} - {W_2}$
Now substitute the values we have,
Therefore, 90 – 30 = ${Q_2}$ – 20
Now simplify this we have,
$ \Rightarrow {Q_2}$ = 90 – 30 + 20 = 60 + 20 = 80 J
So the amount of heat absorbed along the path ADC is 80 J.
So this is the required answer.
Note – In this question the P-V depicts a closed loop it is generally shown for the process for which there is eventually no change in the state of the system even after the completion of the process. So let us suppose that the process started from a certain point A then after the completion it ends up at point A only. Since every system is composed of molecules thus internal energy is due to the individual energy of the molecules only.
Step-By-Step answer:
Given data:
Heat absorbed (${Q_1}$) along the path ABC is 90 J.
$ \Rightarrow {Q_1} = 90J$
And the amount of work done (${W_1}$) by the system along the path ABC is 30 J.
$ \Rightarrow {W_1} = 30J$
And the amount of work done (${W_2}$) by the system along the path ADC is 20 J.
$ \Rightarrow {W_2} = 20J$
Let the amount of heat absorbed along the path ADC be (${Q_2}$)
Now according to the first law of thermodynamics change in internal energy is always constant and it is equal to the difference of heat absorbed along the path and the amount of work done along the same path.
$ \Rightarrow \Delta U$= Q – W
Where, $\Delta U$ = change in internal energy, Q = heat added or absorbed and W = work done.
Therefore change in internal energy along the path ABC is equal to the change in internal energy along the path ADC.
Let change in internal energy along the path ABC = $\Delta {U_1} = {Q_1} - {W_1}$
And change in internal energy along the path ADC = $\Delta {U_2} = {Q_2} - {W_2}$
Therefore, $\Delta {U_1} = \Delta {U_2}$
$ \Rightarrow {Q_1} - {W_1} = {Q_2} - {W_2}$
Now substitute the values we have,
Therefore, 90 – 30 = ${Q_2}$ – 20
Now simplify this we have,
$ \Rightarrow {Q_2}$ = 90 – 30 + 20 = 60 + 20 = 80 J
So the amount of heat absorbed along the path ADC is 80 J.
So this is the required answer.
Note – In this question the P-V depicts a closed loop it is generally shown for the process for which there is eventually no change in the state of the system even after the completion of the process. So let us suppose that the process started from a certain point A then after the completion it ends up at point A only. Since every system is composed of molecules thus internal energy is due to the individual energy of the molecules only.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




