
Assertion:
[10]Annulene is not aromatic though it contains Huckel number of π electrons
Reason:
Steric interaction between internal hydrogens makes it non-polar.
A.Both assertion and reason are correct and the reason is the correct explanation of assertion.
B.Both assertion and reason are correct but the reason is not the correct explanation of assertion.
C.The assertion is correct but the reason is incorrect.
D.Both assertions and reasons are incorrect.
Answer
579.9k+ views
Hint: Annulenes containing even numbers of carbon atoms are completely conjugated monocyclic polyenes having the general formula for annulenes is ${(CH = CH)_n}$ , where n=2,3,4… etc.
Complete step by step answer:
The sufficient and necessary condition for a molecule to be aromatic is given below:
1.There should be a single cyclic cloud of delocalized π electrons above and below the plane of the molecules.
2.It should be a planner to complete delocalization of π electrons
3.It should contain huckle electrons i.e. $(4n + 2)\pi $ electrons where $n = 0,1,2,3.....$etc.
The structure of annulene is given as:
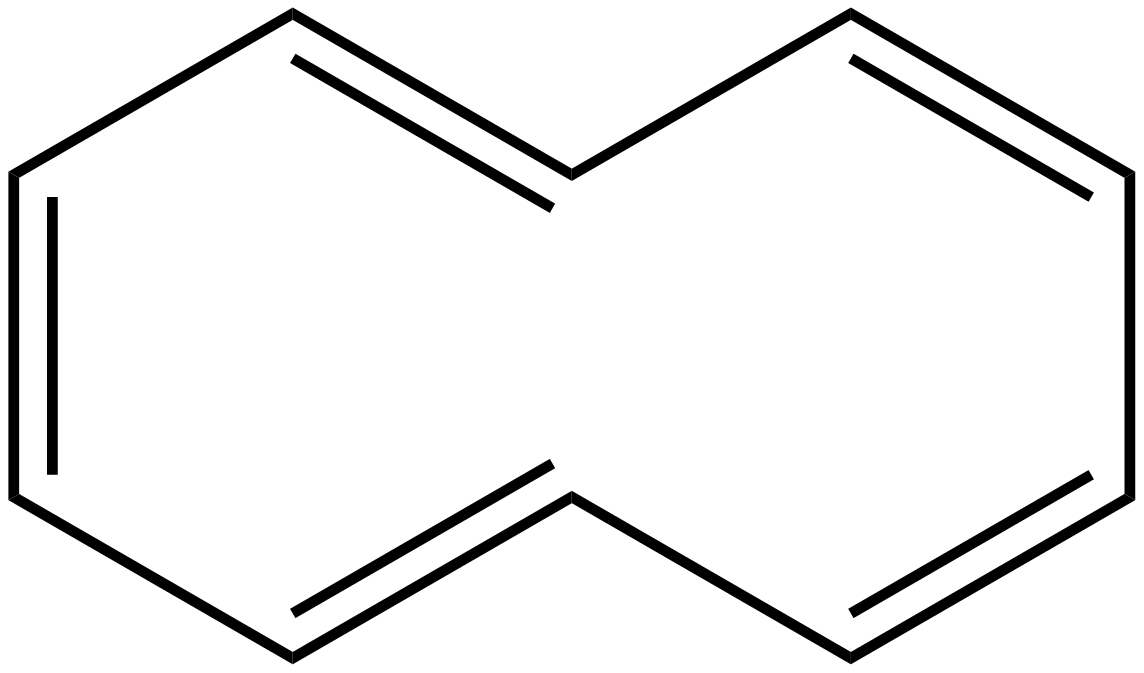
In the above structure we can see there are 10 pi electrons are present hence according to the Huckel rule aromaticity of a compound is found as:
$(4n + 2)\pi = 10\pi $
In the above structure, there are 10 pi electrons are present hence
$ \Rightarrow (4n + 2) = 10$
$ \Rightarrow 4n = 10 - 2$
$ \Rightarrow 4n = 8$
$ \Rightarrow n = \frac{8}{4} = 2$
Annulene an organic compound is a conjugated 10 pi-electron cyclic system according to the huckel rule should display aromaticity. But in actual practice, it is nonaromatic because of various types of ring strains that destabilize all planner geometry. Thus we can say that the reason is the correctly explained assertion and both reason and assertion are true.
Hence the correct answer is option A.
Additional information: According to the IUPAC naming convention, annulenes with seven or more than seven carbon atoms are named as [n] annulene, where n describes the number of a carbon atom in the ring. Annulene may be aromatic, non aromatic, or antiaromatic.
Note:
The [10] annulene is also known as cyclodecapentaene. This is an unstable annulene. The annulene compound is obtained by the photolysis of cis-9,10-dihydronaphthaleneas a mixture of isomers.
Complete step by step answer:
The sufficient and necessary condition for a molecule to be aromatic is given below:
1.There should be a single cyclic cloud of delocalized π electrons above and below the plane of the molecules.
2.It should be a planner to complete delocalization of π electrons
3.It should contain huckle electrons i.e. $(4n + 2)\pi $ electrons where $n = 0,1,2,3.....$etc.
The structure of annulene is given as:

In the above structure we can see there are 10 pi electrons are present hence according to the Huckel rule aromaticity of a compound is found as:
$(4n + 2)\pi = 10\pi $
In the above structure, there are 10 pi electrons are present hence
$ \Rightarrow (4n + 2) = 10$
$ \Rightarrow 4n = 10 - 2$
$ \Rightarrow 4n = 8$
$ \Rightarrow n = \frac{8}{4} = 2$
Annulene an organic compound is a conjugated 10 pi-electron cyclic system according to the huckel rule should display aromaticity. But in actual practice, it is nonaromatic because of various types of ring strains that destabilize all planner geometry. Thus we can say that the reason is the correctly explained assertion and both reason and assertion are true.
Hence the correct answer is option A.
Additional information: According to the IUPAC naming convention, annulenes with seven or more than seven carbon atoms are named as [n] annulene, where n describes the number of a carbon atom in the ring. Annulene may be aromatic, non aromatic, or antiaromatic.
Note:
The [10] annulene is also known as cyclodecapentaene. This is an unstable annulene. The annulene compound is obtained by the photolysis of cis-9,10-dihydronaphthaleneas a mixture of isomers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




