
Assertion: Chemical formula of n-propane and isopropane is the same.
Reason: n-propane does not exhibit chain isomerism.
A.Both assertion and reason are correct and reason is the correct explanation of assertion.
B.Both assertion and reason are correct but reason is not the correct explanation for assertion.
C.Assertion is incorrect but reason is correct.
D.Both assertion and reason are incorrect.
Answer
583.8k+ views
Hint: The name propane gives us an idea that three carbon atoms are attached to each other through single bonds. If two molecules have the same formula but different carbon in the main carbon chain, these are called chain isomerism i.e. difference in the carbon skeleton.
Complete step-by-step answer:
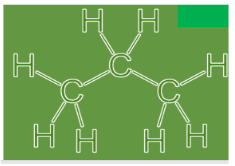
In the word propane, prop means three carbon atoms and ane means single bonds or saturated compounds. If such a compound is drawn, we get the formula having three carbon atoms and eight hydrogen atoms so as to complete the octet of each carbon and make it a neutral molecule.
So, the formula of very basic n-propane comes out to be \[{C_3}{H_8}\] , where n means it is not branched. Let us now look at the compound known as isopropane. We can say that isopropane on itself cannot exist as it would be called propane only. We can see that there are only three carbon which means that if we want an isomer of propane, we need to have a branch on it. But no such branch exists, so it is the same as propane with the same formula and structure \[{C_3}{H_8}\] .
From this, we can say that assertion is true. Let us look at the reason which states that n-propane does not exhibit chain isomerism. For chain isomerism, there should be more than or equal to 4 carbon atoms and therefore, propane can't show chain isomerism. In propane, no matter how we arrange the carbon atoms, we cannot form a new structure i.e. however we number the carbon atoms we will always end up with 3 as the total number of carbons. Thus, the reason is true and very much appropriate for the assertion provided.
Hence the correct option is (A).
Additional information: IUPAC is the abbreviation for the International Union of Pure and Applied Chemistry and it is an organization which is responsible for naming of the compounds. While naming organic compounds, we should remember the IUPAC nomenclature as it is followed internationally.
Note: Also, we can say that the branching cannot be done from the first or the last carbon atom of the structure because the structural formula of propane shows that it does not have sufficient number of carbon atoms to exist in the form of branched isomers. That is why it cannot exhibit structural isomerism.
Complete step-by-step answer:
In the word propane, prop means three carbon atoms and ane means single bonds or saturated compounds. If such a compound is drawn, we get the formula having three carbon atoms and eight hydrogen atoms so as to complete the octet of each carbon and make it a neutral molecule.
So, the formula of very basic n-propane comes out to be \[{C_3}{H_8}\] , where n means it is not branched. Let us now look at the compound known as isopropane. We can say that isopropane on itself cannot exist as it would be called propane only. We can see that there are only three carbon which means that if we want an isomer of propane, we need to have a branch on it. But no such branch exists, so it is the same as propane with the same formula and structure \[{C_3}{H_8}\] .
From this, we can say that assertion is true. Let us look at the reason which states that n-propane does not exhibit chain isomerism. For chain isomerism, there should be more than or equal to 4 carbon atoms and therefore, propane can't show chain isomerism. In propane, no matter how we arrange the carbon atoms, we cannot form a new structure i.e. however we number the carbon atoms we will always end up with 3 as the total number of carbons. Thus, the reason is true and very much appropriate for the assertion provided.
Hence the correct option is (A).

Additional information: IUPAC is the abbreviation for the International Union of Pure and Applied Chemistry and it is an organization which is responsible for naming of the compounds. While naming organic compounds, we should remember the IUPAC nomenclature as it is followed internationally.
Note: Also, we can say that the branching cannot be done from the first or the last carbon atom of the structure because the structural formula of propane shows that it does not have sufficient number of carbon atoms to exist in the form of branched isomers. That is why it cannot exhibit structural isomerism.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




