
Assertion - Oxygen is collected by the downward displacement of water and not over air.
Reason - Downward displacement of water is used to collect oxygen. Any gas can be collected by this method and the gas pushes the water down as it is lighter and doesn’t react with water.
A.Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
B.Both Assertion and Reason are correct and Reason is not the correct explanation for Assertion.
C.Assertion is correct but Reason is incorrect.
D.Both Assertion and Reason are incorrect.
Answer
598.2k+ views
Hint: To answer this question you should know two properties of oxygen. First, it is slightly soluble in water and second, it is slightly heavier than air. Now try to answer this accordingly.
Complete step by step answer:
Oxygen is collected by the downward displacement of water because it is only slightly soluble in water and is less dense than water. Therefore, it can be collected over water without the fear of excessive dilution. It cannot be collected by downward displacement of air since oxygen is slightly heavier than air and also it will get mixed with other gases in the air.
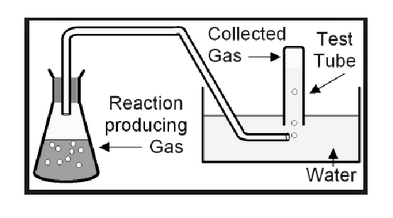
Water displacement is a method of collecting gases that are not soluble in water. These gases can be oxygen, hydrogen, etc. You generate oxygen from a separate container and collect it by filling your collecting bottle with water. The generated gas will now replace the water since no two bodies can occupy the same space at the same time.
Hence, Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
Therefore, we can conclude that the correct answer to this question is option A.
Note: We should know some other important points -
Upward displacement of air - a collection of a gas/liquid below air (i.e. by displacing air upwards)
Downward displacement of water - collection of an immiscible liquid/gas above the surface of the water (i.e. by displacing water downward)
Even though hydrogen is lighter than air it is not collected by downward displacement of air because of these reasons:Hydrogen gets oxidized when it is exposed to oxygen which makes it burn with a pale blue flame and the residue is water. This means that it mixes with oxygen. Downward displacement of air requires a gas that does not mix with air and is lighter than air. Hydrogen is lighter than air but mixes with the air.
Complete step by step answer:
Oxygen is collected by the downward displacement of water because it is only slightly soluble in water and is less dense than water. Therefore, it can be collected over water without the fear of excessive dilution. It cannot be collected by downward displacement of air since oxygen is slightly heavier than air and also it will get mixed with other gases in the air.

Water displacement is a method of collecting gases that are not soluble in water. These gases can be oxygen, hydrogen, etc. You generate oxygen from a separate container and collect it by filling your collecting bottle with water. The generated gas will now replace the water since no two bodies can occupy the same space at the same time.
Hence, Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
Therefore, we can conclude that the correct answer to this question is option A.
Note: We should know some other important points -
Upward displacement of air - a collection of a gas/liquid below air (i.e. by displacing air upwards)
Downward displacement of water - collection of an immiscible liquid/gas above the surface of the water (i.e. by displacing water downward)
Even though hydrogen is lighter than air it is not collected by downward displacement of air because of these reasons:Hydrogen gets oxidized when it is exposed to oxygen which makes it burn with a pale blue flame and the residue is water. This means that it mixes with oxygen. Downward displacement of air requires a gas that does not mix with air and is lighter than air. Hydrogen is lighter than air but mixes with the air.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




