
Assertion: The micelle formation by sodium stearate in water has $ - CO{O^ - }$ groups at the surface.
Reason: Surface tension of water is reduced by the addition of stearate.
A. Both assertion and reason are correct and reason is the correct explanation of assertion.
B. Both assertion and reason are correct and reason is not the correct explanation of assertion.
C. Assertion is correct but reason is incorrect.
D. Assertion is incorrect but reason is correct.
Answer
576.6k+ views
Hint: To answer this question, you should recall the concept of micelle formation and the properties of micelles. Sodium stearate has an ionic head attached to a long covalent organic tail.
Complete step by step solution:
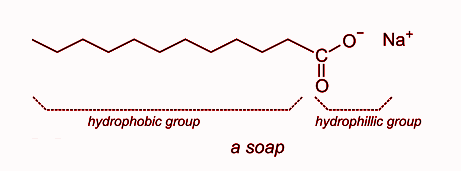
When dissolved in water, sodium stearate dissociates into $RCO{O^ - }$ and $N{a^ + }$ ions.
The$RCO{O^ - }$ ions consist of two parts: a long hydrocarbon chain ($R$) which is hydrophobic and a polar carboxylate group ($CO{O^ - }$) which is hydrophilic.
We know that, when concentration of the ions is increased and it reaches a critical micelle concentration, the anions begin to aggregate.
The ions aggregate to form a spherical shape with their hydrocarbon chains (hydrophobic) pointing towards the centre of the sphere and the carboxylate ion part (hydrophilic) remaining outward on the surface of the sphere. The aggregate formed is known as ionic micelle.
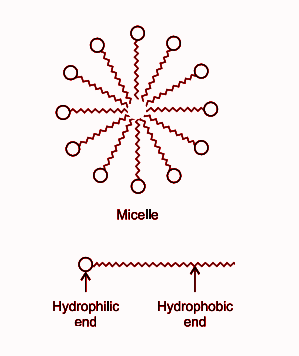
As you can see in the structure of micelle, the carboxylate groups are attached to water and are at the surface.
Thus, we can infer that the assertion is true.
Also, it is true that due to the addition of stearate, the surface tension of water reduces.
The stearate ions replace some of the water molecules at the surface. As a result, the contraction forces at the surface are reduced and surface tension decreases.
But, the decrease in surface tension is not responsible for the presence of the carboxylate group at the surface. The presence of $CO{O^ - }$ at the surface is due to the micelle formation
Hence, both assertion and reason are correct but the reason is not the correct explanation of assertion
The correct option is B.
Note: This property of sodium salts of long chain fatty acids to form micelles has its application in cleansing action of soap. The soap molecules form micelle around oil droplets with the hydrophobic part in the oil droplet and hydrophilic part projecting outwards. As the polar group interacts with water, the oil droplet surrounded by the ions is pulled into the water removing it from the dirty surface.
Complete step by step solution:

When dissolved in water, sodium stearate dissociates into $RCO{O^ - }$ and $N{a^ + }$ ions.
The$RCO{O^ - }$ ions consist of two parts: a long hydrocarbon chain ($R$) which is hydrophobic and a polar carboxylate group ($CO{O^ - }$) which is hydrophilic.
We know that, when concentration of the ions is increased and it reaches a critical micelle concentration, the anions begin to aggregate.
The ions aggregate to form a spherical shape with their hydrocarbon chains (hydrophobic) pointing towards the centre of the sphere and the carboxylate ion part (hydrophilic) remaining outward on the surface of the sphere. The aggregate formed is known as ionic micelle.

As you can see in the structure of micelle, the carboxylate groups are attached to water and are at the surface.
Thus, we can infer that the assertion is true.
Also, it is true that due to the addition of stearate, the surface tension of water reduces.
The stearate ions replace some of the water molecules at the surface. As a result, the contraction forces at the surface are reduced and surface tension decreases.
But, the decrease in surface tension is not responsible for the presence of the carboxylate group at the surface. The presence of $CO{O^ - }$ at the surface is due to the micelle formation
Hence, both assertion and reason are correct but the reason is not the correct explanation of assertion
The correct option is B.
Note: This property of sodium salts of long chain fatty acids to form micelles has its application in cleansing action of soap. The soap molecules form micelle around oil droplets with the hydrophobic part in the oil droplet and hydrophilic part projecting outwards. As the polar group interacts with water, the oil droplet surrounded by the ions is pulled into the water removing it from the dirty surface.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




