
Assertion-Osmosis is a special type of diffusion of water through a semipermeable membrane.
Reason-The net direction and rate of osmosis depend only on the pressure gradient.
(a)Both assertion and reason are correct and the reason is the correct explanation for assertion
(b)Both assertion and reason are correct but the reason is not the correct explanation for assertion
(c)Assertion is correct but the reason is incorrect
(d)Both assertion and reason are correct.
Answer
582.9k+ views
Hint: Osmosis in the process of diffusion of molecules from a lower concentration solution to another higher concentration solution. There are several factors affecting the rate and direction of osmosis in a certain system.
Complete answer:
Osmosis is a special process of diffusion of solute to a solution through a semipermeable membrane. Here two factors determine the net direction and rate. One of them is the pressure gradient whereas the other is the concentration gradient. The difference in the water potential of solution separated by the semi-permeable membrane determines the net force or gradient.
Additional Information: Osmosis is another type of diffusion. It is the diffusion of molecules of a solvent from a solution to another solution with higher concentration through a semipermeable membrane. It is a passive process and happens without the use of energy.
Here’s a representation of osmosis and the osmotic pressure
The factors that affect the rate of osmosis are listed below
-Osmotic gradient: It is the difference of concentration between two solutions that are on either side of the membrane. It is used to compare solutions, allowing water to diffuse towards the hypertonic solution. When equilibrium is established, the water flows in both sides equally and stabilizes the solution.
-Osmotic pressure: Increasing the pressure in the region of a higher concentration, we can oppose the osmosis of molecules. The required force per unit area i.e. pressure applied on the higher concentration side to prevent the flow of water through the semipermeable membrane is equivalent to the osmotic pressure of the solution. It is a colligative property.
So, the correct answer is ‘assertion is correct but the reason is incorrect’.
Note: Other factors such as temperature, surface area, water potential, etc. may also affect the rate and direction of osmosis. With an increase in temperature, the rate of osmosis increases. Molecules can move easily across a larger surface area. The rate of osmosis gets faster with the increase in water potential.
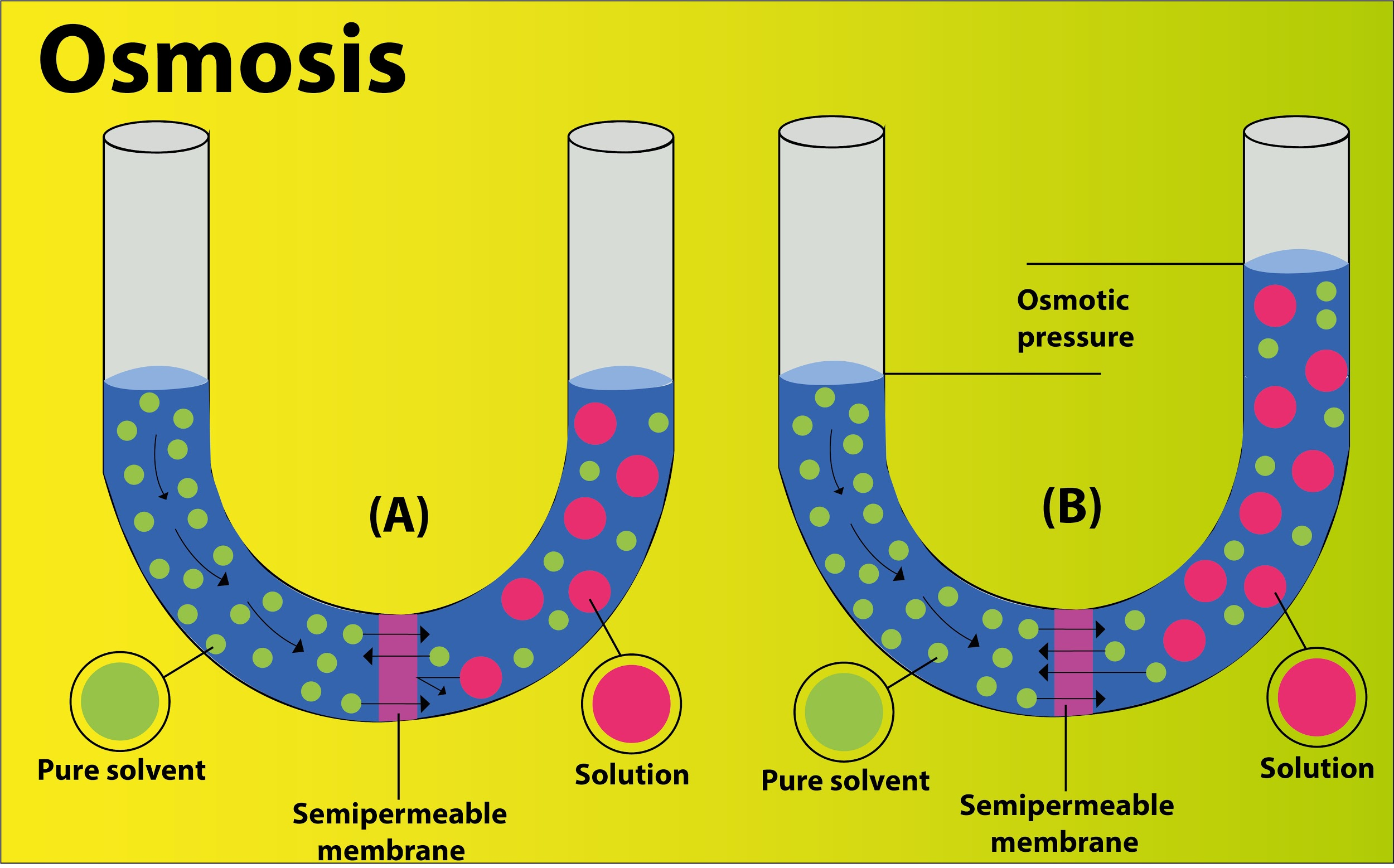
Complete answer:
Osmosis is a special process of diffusion of solute to a solution through a semipermeable membrane. Here two factors determine the net direction and rate. One of them is the pressure gradient whereas the other is the concentration gradient. The difference in the water potential of solution separated by the semi-permeable membrane determines the net force or gradient.
Additional Information: Osmosis is another type of diffusion. It is the diffusion of molecules of a solvent from a solution to another solution with higher concentration through a semipermeable membrane. It is a passive process and happens without the use of energy.
Here’s a representation of osmosis and the osmotic pressure
The factors that affect the rate of osmosis are listed below
-Osmotic gradient: It is the difference of concentration between two solutions that are on either side of the membrane. It is used to compare solutions, allowing water to diffuse towards the hypertonic solution. When equilibrium is established, the water flows in both sides equally and stabilizes the solution.
-Osmotic pressure: Increasing the pressure in the region of a higher concentration, we can oppose the osmosis of molecules. The required force per unit area i.e. pressure applied on the higher concentration side to prevent the flow of water through the semipermeable membrane is equivalent to the osmotic pressure of the solution. It is a colligative property.
So, the correct answer is ‘assertion is correct but the reason is incorrect’.
Note: Other factors such as temperature, surface area, water potential, etc. may also affect the rate and direction of osmosis. With an increase in temperature, the rate of osmosis increases. Molecules can move easily across a larger surface area. The rate of osmosis gets faster with the increase in water potential.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




