
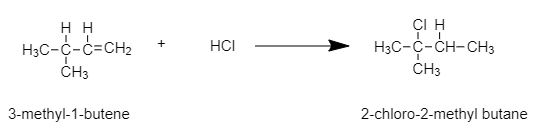
Assertion:The addition of HCl to 3-methyl-1-butene gives 2-chloro-2-methyl butane as the major product.
Reason:Rearrangement of carbocation to move stable carbocation leads to the formation of a major product.
A.Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
B.Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion.
C.Assertion is correct but Reason is incorrect.
D.Both Assertion and Reason are incorrect.
Answer
545.7k+ views
Hint: Addition reaction is a type of reaction in which one compound is added to another to combine and form the product. Along with addition, rearrangement of carbocation takes place, in which the proton that is H atom is transferred to form a short-lived intermediate which further can lead to structural rearrangement of the molecule to obtain a larger yield.
Complete step-by-step answer:When an addition reaction is performed on alkene by adding strong acids like HBr, HI, HCl, etc. the C=C bond is broken to form new covalent bonds with the hydrogen and conjugate base of the acid. The general reaction will be,
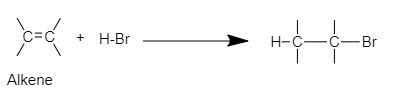
In this example, that is 3-methyl-1-butene, the C involved in double bonds are structurally unsymmetrical, hence a reaction with a strong acid like HCl will give two products in different quantities. This is because of the rearrangement of the carbocation.
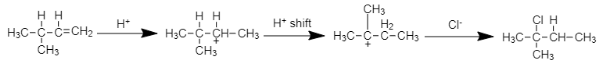
Mechanism of the reaction:
Hence, the correct option is option A - Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
Note:In this reaction that is the reaction of unsymmetrical alkene when reacts with HCl the double bond that is protonated will form a major product fast due to stable carbocation rearrangement compared to the other product.
Complete step-by-step answer:When an addition reaction is performed on alkene by adding strong acids like HBr, HI, HCl, etc. the C=C bond is broken to form new covalent bonds with the hydrogen and conjugate base of the acid. The general reaction will be,

In this example, that is 3-methyl-1-butene, the C involved in double bonds are structurally unsymmetrical, hence a reaction with a strong acid like HCl will give two products in different quantities. This is because of the rearrangement of the carbocation.

Mechanism of the reaction:

Hence, the correct option is option A - Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
Note:In this reaction that is the reaction of unsymmetrical alkene when reacts with HCl the double bond that is protonated will form a major product fast due to stable carbocation rearrangement compared to the other product.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




