
Atomic number of an atom is equal to the ______
A.number of protons
B.number of electrons
C.both a and b
D.sum of proton and electron
Answer
585.6k+ views
Hint: We know that an atom comprises positive charge nucleus, with negatively charged particles called as electrons surrounding the nucleus. We also know that the atomic nucleus consists of protons and neutrons present in them.
Complete step by step answer:
The protons and neutrons are present inside a small, dense region present at the center of the atom called a nucleus.
We know that the neutrons and protons are present inside the nucleus. The neutron and protons are collectively called nucleons.
We know that the atom contains positively charged nucleus present at its center and negatively charged particles known as electrons, which revolves around the nucleus in circulating paths called as orbitals. The nucleus and electrons are bound together by the means of electrostatic force.
The mass of the atom is concentrated in the nucleus with a small part from the cloud of electrons.
The sum of the protons and neutrons in the nucleus is known as mass number.
The number of protons present in the nucleus is known as atomic number.
For an element ${\text{E,}}$ the top left number represents the mass number and the bottom left number represents the atomic number.
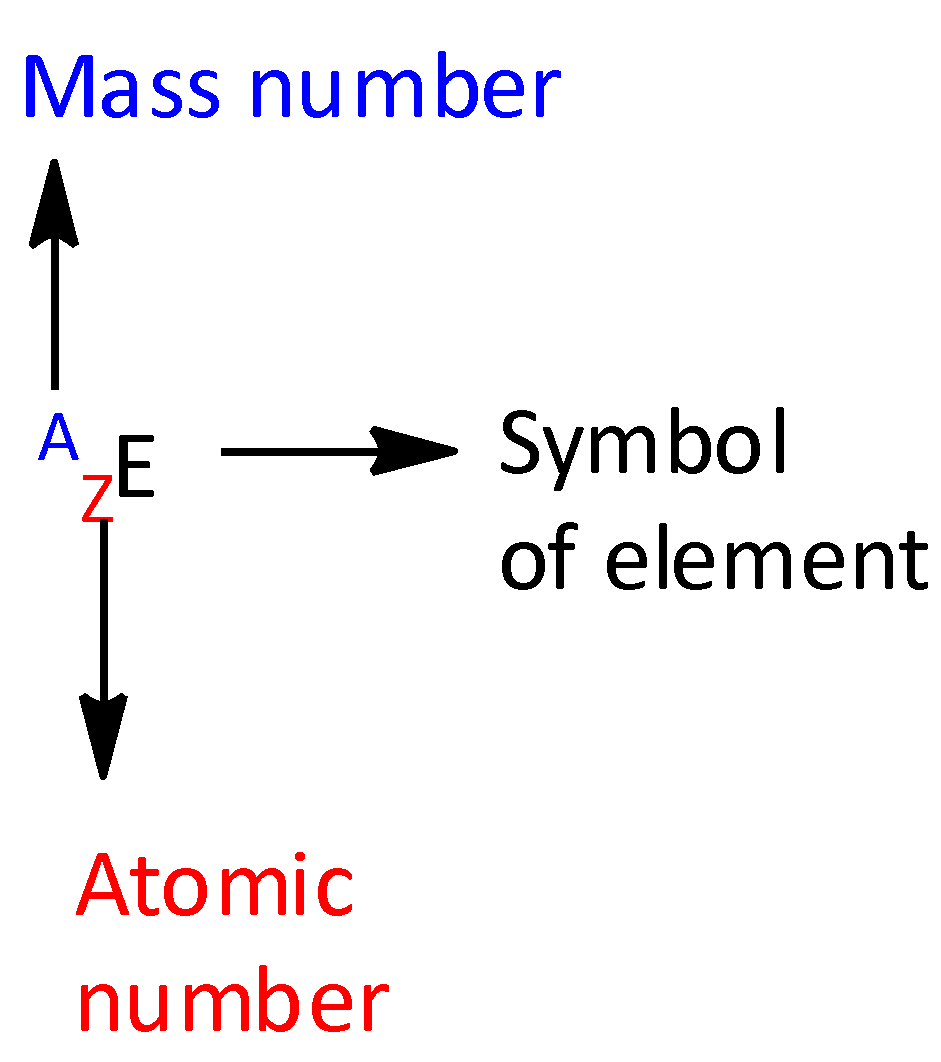
The letter A represents mass number.
The letter Z represents atomic numbers.
Atomic number of an element tells about the number of protons present.
${\text{Atomic}}\,{\text{number = Number}}\,{\text{of}}\,{\text{protons}}$
The number of protons present will be equal to the number of electrons.
${\text{Number}}\,{\text{of}}\,{\text{protons = Number}}\,{\text{of}}\,{\text{electrons}}$
Therefore, the atomic number of an atom is equal to both the number of electrons and protons present in an atom.
$\therefore $ Option (C) is correct .
Note:
Let us take an example of an element having atomic number 12, the number of protons and electrons present in the element will also be 12. Every element in the periodic table will have a different number of protons. Since an atom contains the same number of protons and electrons, we can say an atom is neutral in terms of electrical charge. The atomic number of the element is written front and slightly below the symbol of the element.
Example: For the given element ${}_2^4He$, the atomic number is two and the number of protons and electrons present in the element is also two.
Complete step by step answer:
The protons and neutrons are present inside a small, dense region present at the center of the atom called a nucleus.
We know that the neutrons and protons are present inside the nucleus. The neutron and protons are collectively called nucleons.
We know that the atom contains positively charged nucleus present at its center and negatively charged particles known as electrons, which revolves around the nucleus in circulating paths called as orbitals. The nucleus and electrons are bound together by the means of electrostatic force.
The mass of the atom is concentrated in the nucleus with a small part from the cloud of electrons.
The sum of the protons and neutrons in the nucleus is known as mass number.
The number of protons present in the nucleus is known as atomic number.
For an element ${\text{E,}}$ the top left number represents the mass number and the bottom left number represents the atomic number.

The letter A represents mass number.
The letter Z represents atomic numbers.
Atomic number of an element tells about the number of protons present.
${\text{Atomic}}\,{\text{number = Number}}\,{\text{of}}\,{\text{protons}}$
The number of protons present will be equal to the number of electrons.
${\text{Number}}\,{\text{of}}\,{\text{protons = Number}}\,{\text{of}}\,{\text{electrons}}$
Therefore, the atomic number of an atom is equal to both the number of electrons and protons present in an atom.
$\therefore $ Option (C) is correct .
Note:
Let us take an example of an element having atomic number 12, the number of protons and electrons present in the element will also be 12. Every element in the periodic table will have a different number of protons. Since an atom contains the same number of protons and electrons, we can say an atom is neutral in terms of electrical charge. The atomic number of the element is written front and slightly below the symbol of the element.
Example: For the given element ${}_2^4He$, the atomic number is two and the number of protons and electrons present in the element is also two.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




