
Atomic number of carbon atom is:
A) 1
B) 3
C) 6
D) 8
Answer
588.3k+ views
Hint: The atomic number is equal to the number of protons in the nucleus. In the periodic table, the atomic number increases as we move from left to right and top to bottom. The carbon atom has 6 protons in its nucleus and 6 electrons revolving around the nucleus in an uncharged state.
Complete step by step answer:
The atomic number of an element equals the protons found in the nucleus of an atom. The atomic number of the element is represented by the symbol Z. The atomic number is a unique identity of a chemical element. The Z is equal to the charge number of the nucleus.
$\text{ Atomic number (Z) = Number of proton}$
We know that carbon C is a group 14 element. Each carbon atom has 6 protons and 6 electrons. We know that the atomic number is equal to the number of the protons, therefore the atomic number of carbon is 6.
Carbon plays a crucial role in defining life. Therefore its electronic configuration and its atomic number are of great importance.
Its electronic configuration is as follows
$\text{ 1}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{p}}^{\text{2}}}$
The valence shell of carbon contains the 4 electrons.it forms a covalent bond by sharing its valence electrons.
The elemental representation of carbon is as follows:
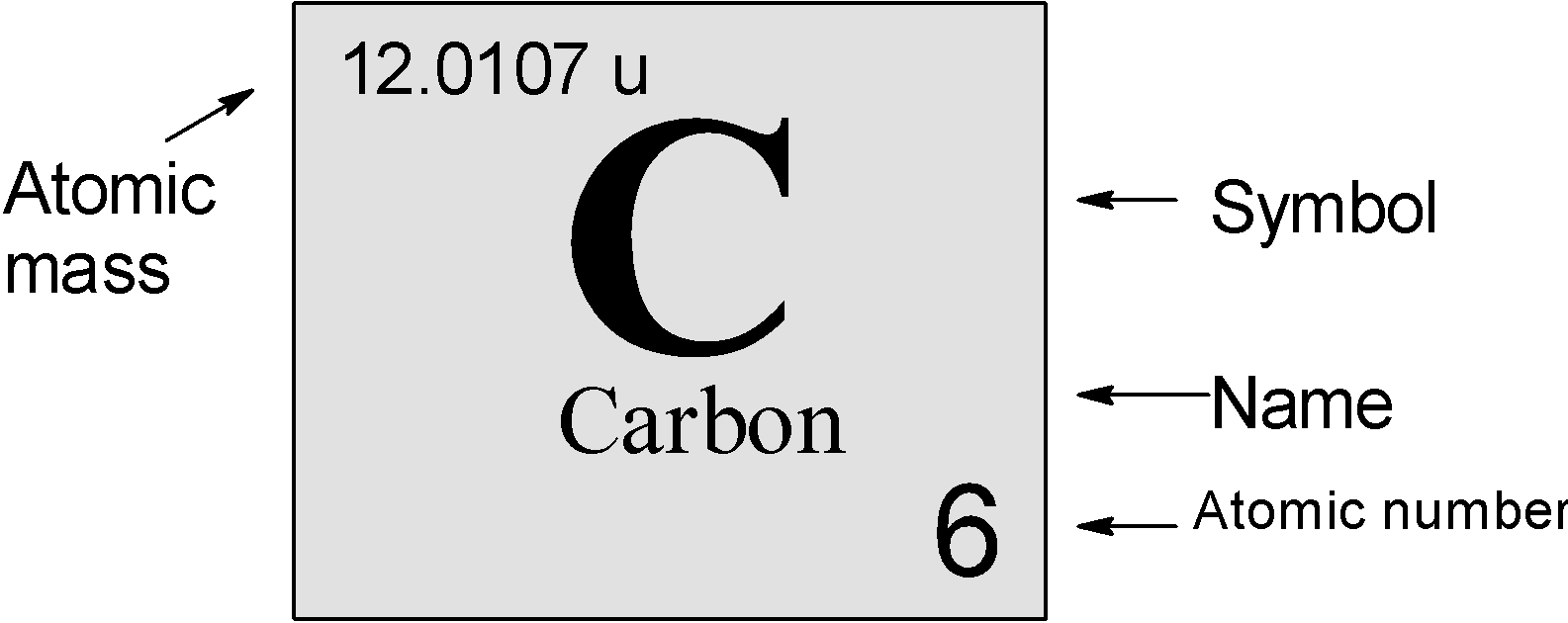
It can be also noted in the standard format $\text{ }_{\text{Z}}^{\text{A}}\text{X}$ , where A is the atomic mass and Z is the atomic number. The carbon is represented as, $\text{ }_{\text{6}}^{\text{12}}\text{C}$.
Therefore carbon has an atomic number of 6.
So, the correct answer is “Option C”.
Additional Information:
The atomic mass of an atom of an element is equal to the sum of neutrons and protons. The atomic mass is represented by A. The formula is,
$\text{Atomic mass (A) = Number of Proton(P) + Number of Proton(N)}$
The carbon has 6 protons and 6 neutrons present in the nucleus which stabilizes the nucleus. Therefore the atomic mass of carbon is 12.
Note: Sometimes we refer to the atomic number as the number of electrons present in an atom of an element. This is because in an uncharged or in zero charge state the total positive charge produced by the proton is nullified by the negatively charged electrons revolving around the nucleus. Thus, the atomic number equals the total number of electrons.
Complete step by step answer:
The atomic number of an element equals the protons found in the nucleus of an atom. The atomic number of the element is represented by the symbol Z. The atomic number is a unique identity of a chemical element. The Z is equal to the charge number of the nucleus.
$\text{ Atomic number (Z) = Number of proton}$
We know that carbon C is a group 14 element. Each carbon atom has 6 protons and 6 electrons. We know that the atomic number is equal to the number of the protons, therefore the atomic number of carbon is 6.
Carbon plays a crucial role in defining life. Therefore its electronic configuration and its atomic number are of great importance.
Its electronic configuration is as follows
$\text{ 1}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{p}}^{\text{2}}}$
The valence shell of carbon contains the 4 electrons.it forms a covalent bond by sharing its valence electrons.
The elemental representation of carbon is as follows:

It can be also noted in the standard format $\text{ }_{\text{Z}}^{\text{A}}\text{X}$ , where A is the atomic mass and Z is the atomic number. The carbon is represented as, $\text{ }_{\text{6}}^{\text{12}}\text{C}$.
Therefore carbon has an atomic number of 6.
So, the correct answer is “Option C”.
Additional Information:
The atomic mass of an atom of an element is equal to the sum of neutrons and protons. The atomic mass is represented by A. The formula is,
$\text{Atomic mass (A) = Number of Proton(P) + Number of Proton(N)}$
The carbon has 6 protons and 6 neutrons present in the nucleus which stabilizes the nucleus. Therefore the atomic mass of carbon is 12.
Note: Sometimes we refer to the atomic number as the number of electrons present in an atom of an element. This is because in an uncharged or in zero charge state the total positive charge produced by the proton is nullified by the negatively charged electrons revolving around the nucleus. Thus, the atomic number equals the total number of electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




