
What is the atomic radius? Why does the atomic radius decrease across a period?
Answer
573.6k+ views
Hint: Atomic radius is used to express the size of an atom. It is a distance between the nucleus and the edge of the outermost orbital. The size of an atom depends on the forces of attraction between the nucleus and the outermost shell electrons. Along the period the number of electrons added to the same shell increases the attraction between the positively charged nucleus and electron and pulls closer to the nucleus.
Complete answer:
The atomic radius of an atom depends on the size of an atom. Atomic radius is the shortest distance between the nuclei of an atom and the outermost shell of an atom.
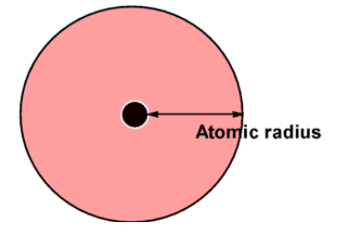
-It is also half a distance between the nucleuses of two identical nuclei bonded to each other.
The atomic radius of an element in the period generally decreases as we move along a period from left to right. The periodic trend of the atomic radius depends on the nuclear charge.
-As we move along the period (from left to right) the extra positive charge is added to the nucleus. That is the atomic number of elements goes on increasing. Similarly, the number of electrons is added into the same shell. Since electrons are added in the same shell the electrons experience a high attractive force. The force of attraction between the nucleus and the outermost electrons to such an extent that nuclear charge pulls in the shell and thus reduces the atomic radii or atom.
-Increasing nuclear charge results in all of the orbital electrons being pulled closer to the nucleus.
-For example, on moving from lithium to beryllium one extra positive charge is added to the nucleus and an extra orbital electron is also added.
-In a given period, alkali metal (or the group 1) elements have a larger atomic radius. There are big atoms. However, as moving further in the period the atomic size decreases to the smallest. Halogen has the smallest atomic radius.
When the period contains the transition elements (d block and f block elements) the contraction of size is larger. Let’s consider second-period elements and their atomic radius.
Thus atomic radius decreases along the period.
Note: Note that there is some exception in the atomic radius along the period. One of the exceptions is oxygen. Atomic radius of oxygen is slightly greater than the nitrogen (it is approximately equal to the atomic size of nitrogen).similar in a period the atomic size of Noble gas is greater than halogen. This is because noble gas is held together by the van der Waals forces.
Complete answer:
The atomic radius of an atom depends on the size of an atom. Atomic radius is the shortest distance between the nuclei of an atom and the outermost shell of an atom.

-It is also half a distance between the nucleuses of two identical nuclei bonded to each other.
The atomic radius of an element in the period generally decreases as we move along a period from left to right. The periodic trend of the atomic radius depends on the nuclear charge.
-As we move along the period (from left to right) the extra positive charge is added to the nucleus. That is the atomic number of elements goes on increasing. Similarly, the number of electrons is added into the same shell. Since electrons are added in the same shell the electrons experience a high attractive force. The force of attraction between the nucleus and the outermost electrons to such an extent that nuclear charge pulls in the shell and thus reduces the atomic radii or atom.
-Increasing nuclear charge results in all of the orbital electrons being pulled closer to the nucleus.
-For example, on moving from lithium to beryllium one extra positive charge is added to the nucleus and an extra orbital electron is also added.
-In a given period, alkali metal (or the group 1) elements have a larger atomic radius. There are big atoms. However, as moving further in the period the atomic size decreases to the smallest. Halogen has the smallest atomic radius.
When the period contains the transition elements (d block and f block elements) the contraction of size is larger. Let’s consider second-period elements and their atomic radius.
| Element | $\text{ Li }$ | $\text{ Be }$ | $\text{ B }$ | $\text{ C }$ | $\text{ N }$ | $\text{ O }$ | $\text{ F }$ |
| Atomic radii | $\text{ 1}\text{.23 }$ | $\text{ 0}\text{.89 }$ | $\text{ 0}\text{.80 }$ | $\text{ 0}\text{.77 }$ | $\text{ 0}\text{.74 }$ | $\text{ 0}\text{.74 }$ | $\text{ 0}\text{.72 }$ |
Thus atomic radius decreases along the period.
Note: Note that there is some exception in the atomic radius along the period. One of the exceptions is oxygen. Atomic radius of oxygen is slightly greater than the nitrogen (it is approximately equal to the atomic size of nitrogen).similar in a period the atomic size of Noble gas is greater than halogen. This is because noble gas is held together by the van der Waals forces.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




