
Average atomic mass of Mg is approximately:
(A) 25.0
(B) 24.5
(C) 25.2
(D) 25.8

Answer
591.3k+ views
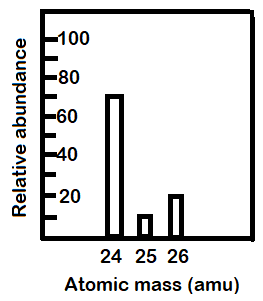
Hint: The graph gives us abundances of different isotopes of magnesium. The average atomic weight of any element is the sum of the masses of the isotopes multiplied with their respective percentage abundances.
Formula used:
-Average atomic weight:
= \[\sum\limits_{i = 1}^n {(mas{s_{(i)}})(abundanc{e_{(i)}})} \] (1)
Where, mass = atomic weight of the isotope
Abundance = percentage abundance in which the respective isotope is present.
Complete answer:
-Isotopes are those atoms of a chemical element which have the same atomic number, same position in the periodic table and nearly the same chemical properties but they differ in the mass number and physical properties.
Isotopes of an element have the same number of electrons and protons but differ in the number of neutrons.
Example: -Carbon has 3 isotopes: C-12, C-13, C-14
-Hydrogen has 3 isotopes: H-1, D-2, T-3
-Bromine has 2 stable isotopes: Br-79, Br-81
-Magnesium has 3 isotopes: Mg-24, Mg-25, Mg-26
-From the graph given in the question we can tell the relative abundances of each isotope. They will be: (a) Mg-24: 70%
(b) Mg-25: 10%
(c) Mg-26: 20%
We use equation (1) to calculate the average atomic mass of Mg atom:
Average atomic weight=$\sum\limits_{i = 1}^n {(mas{s_{(i)}})(abundanc{e_{(i)}})} $
= $\left\{ {24 \times \frac{{70}}{{100}}} \right\} + \left\{ {25 \times \frac{{10}}{{100}}} \right\} + \left\{ {26 \times \frac{{20}}{{100}}} \right\}$
= 16.8 + 2.5 + 5.2
= 24.5
Average atomic weight of Mg is 24.5 amu.
So, the correct option will be: (B) 24.5
Note:
Isotopes are those which have the same atomic number but different mass number. Isobars are those atoms which have the same mass number but different atomic number. Isotones are those which have the same neutron number but different proton number. Don’t confuse them with each other.
Formula used:
-Average atomic weight:
= \[\sum\limits_{i = 1}^n {(mas{s_{(i)}})(abundanc{e_{(i)}})} \] (1)
Where, mass = atomic weight of the isotope
Abundance = percentage abundance in which the respective isotope is present.
Complete answer:
-Isotopes are those atoms of a chemical element which have the same atomic number, same position in the periodic table and nearly the same chemical properties but they differ in the mass number and physical properties.
Isotopes of an element have the same number of electrons and protons but differ in the number of neutrons.
Example: -Carbon has 3 isotopes: C-12, C-13, C-14
-Hydrogen has 3 isotopes: H-1, D-2, T-3
-Bromine has 2 stable isotopes: Br-79, Br-81
-Magnesium has 3 isotopes: Mg-24, Mg-25, Mg-26
-From the graph given in the question we can tell the relative abundances of each isotope. They will be: (a) Mg-24: 70%
(b) Mg-25: 10%
(c) Mg-26: 20%
We use equation (1) to calculate the average atomic mass of Mg atom:
Average atomic weight=$\sum\limits_{i = 1}^n {(mas{s_{(i)}})(abundanc{e_{(i)}})} $
= $\left\{ {24 \times \frac{{70}}{{100}}} \right\} + \left\{ {25 \times \frac{{10}}{{100}}} \right\} + \left\{ {26 \times \frac{{20}}{{100}}} \right\}$
= 16.8 + 2.5 + 5.2
= 24.5
Average atomic weight of Mg is 24.5 amu.
So, the correct option will be: (B) 24.5
Note:
Isotopes are those which have the same atomic number but different mass number. Isobars are those atoms which have the same mass number but different atomic number. Isotones are those which have the same neutron number but different proton number. Don’t confuse them with each other.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




