
What is (B) in the following sequence of reactions?
(A)
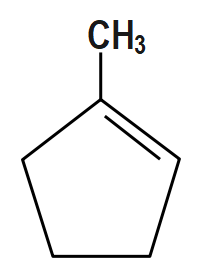
(B)
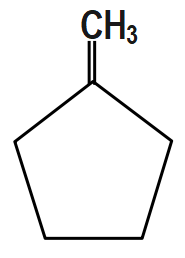
(C) $ C{{H}_{3}}-CH=CH-C{{H}_{2}}C{{H}_{2}}C{{H}_{3}} $
(D) $ C{{H}_{3}}-C(=O)-C{{H}^{2}}C{{H}_{2}}C{{H}_{2}}{{C}_{4}}{{H}_{3}} $


Answer
527.1k+ views
Hint :We know that in order to know how to solve a stoichiometry problem, we must first know what a stoichiometry is. Stoichiometry is the relationship between the quantities of the reactants and the products. If we know the quantity of the reactant it will be easy to determine the product.
Complete Step By Step Answer:
Let us understand deeply about stoichiometry. Stoichiometry will be based on the Law of conservation of mass. That means that the total mass of the reactants will be equal to the total mass of the products. If we know the amount of the reactants, from that we can calculate the amount of the product. We can determine the quantity of the product empirically from the quantity of the reactant.
We have to remember that there are four steps for solving a stoichiometric problem.
First thing is that we have to write a balanced equation. We have to always keep in mind that the constituent part of a chemical reaction is neither destroyed nor lost. The reactions yield must correspond to the reactant.
The next step is to convert the unit into the moles. When a unit is converted into moles it will involve the conversion factor. The main aim of the conversion factor is to convert units such as the grams, volume of a gas into moles or vice versa.
Now by using the mole ratio, we have to calculate the other substance (product).
Here, First step is an example of the Wittig reaction and then in addition to the base $ Bu-Li, $ it gives a thermodynamic product.
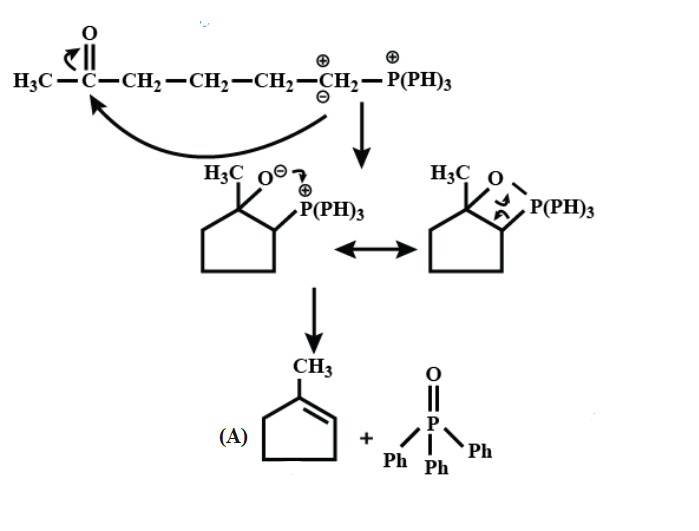
On further continuing the reaction;
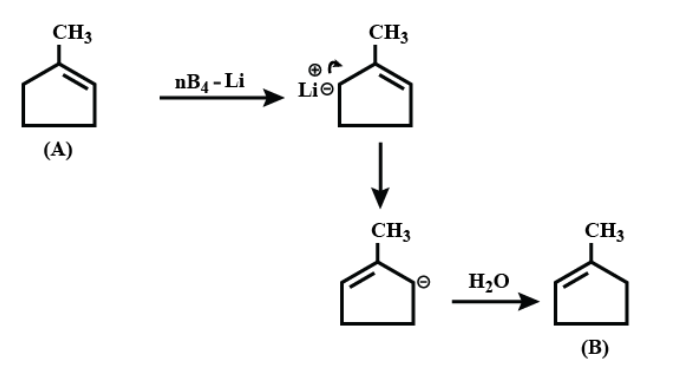
Therefore the correct answer is option A.
Note :
Remember that we have to remember that stoichiometry compounds and non-stoichiometry compounds are different from one another. Non-stoichiometry compound is mostly an inorganic compound whose proportions of the elemental composition cannot be determined by the ratio of the natural number.
Complete Step By Step Answer:
Let us understand deeply about stoichiometry. Stoichiometry will be based on the Law of conservation of mass. That means that the total mass of the reactants will be equal to the total mass of the products. If we know the amount of the reactants, from that we can calculate the amount of the product. We can determine the quantity of the product empirically from the quantity of the reactant.
We have to remember that there are four steps for solving a stoichiometric problem.
First thing is that we have to write a balanced equation. We have to always keep in mind that the constituent part of a chemical reaction is neither destroyed nor lost. The reactions yield must correspond to the reactant.
The next step is to convert the unit into the moles. When a unit is converted into moles it will involve the conversion factor. The main aim of the conversion factor is to convert units such as the grams, volume of a gas into moles or vice versa.
Now by using the mole ratio, we have to calculate the other substance (product).
Here, First step is an example of the Wittig reaction and then in addition to the base $ Bu-Li, $ it gives a thermodynamic product.

On further continuing the reaction;

Therefore the correct answer is option A.
Note :
Remember that we have to remember that stoichiometry compounds and non-stoichiometry compounds are different from one another. Non-stoichiometry compound is mostly an inorganic compound whose proportions of the elemental composition cannot be determined by the ratio of the natural number.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




