
Bakelite is obtained from phenol by reacting it with:
A) ${\left( {C{H_2}OH} \right)_2}$
B) $C{H_3}CHO$
C) $C{H_3}COC{H_3}$
D) $HCHO$
Answer
546.6k+ views
Hint:
To answer this question, you must recall the formula and preparation of bakelite. Bakelite is a phenol resin and is a highly cross linked material which is formed by chemical reactions under the action of heat, catalyst, UV light, etc.
Complete step by step solution:
Bakelite is a polymer made up of synthetic components and comes under the category of plastic polymers. It is a three dimensional cross linked polymer and is made up of the monomer units, phenol and formaldehyde.
Bakelite is prepared by the reaction of phenol and formaldehyde. Its preparation occurs in two steps. The first step involves the reaction of formaldehyde with phenol to produce ortho- hydroxyl methyl phenol. In the second step, the ortho- hydroxyl methyl phenol polymerizes with phenol to form bakelite as a product. This polymerization involves a condensation reaction as the two molecules come together and lose a water molecule.
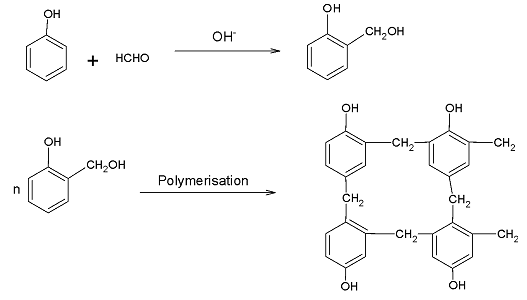
Overall, formaldehyde and phenol condense forming the monomer for bakelite giving out water as a side product. When this reaction occurs at a large scale, multiple units are formed which come together and form a chain like structure and bakelite expands its size. Hence, bakelite is also known as a co- polymer.
Thus, the correct option is D.
Note:
If you do not know the structure of a bakelite, you can predict the other monomer by observing a bakelite molecule. We can see that there is one carbon atom present between two phenyl rings. Hence we can conclude that formaldehyde is used in the reaction. This trick can be used to answer a number of similar problems.
To answer this question, you must recall the formula and preparation of bakelite. Bakelite is a phenol resin and is a highly cross linked material which is formed by chemical reactions under the action of heat, catalyst, UV light, etc.
Complete step by step solution:
Bakelite is a polymer made up of synthetic components and comes under the category of plastic polymers. It is a three dimensional cross linked polymer and is made up of the monomer units, phenol and formaldehyde.
Bakelite is prepared by the reaction of phenol and formaldehyde. Its preparation occurs in two steps. The first step involves the reaction of formaldehyde with phenol to produce ortho- hydroxyl methyl phenol. In the second step, the ortho- hydroxyl methyl phenol polymerizes with phenol to form bakelite as a product. This polymerization involves a condensation reaction as the two molecules come together and lose a water molecule.

Overall, formaldehyde and phenol condense forming the monomer for bakelite giving out water as a side product. When this reaction occurs at a large scale, multiple units are formed which come together and form a chain like structure and bakelite expands its size. Hence, bakelite is also known as a co- polymer.
Thus, the correct option is D.
Note:
If you do not know the structure of a bakelite, you can predict the other monomer by observing a bakelite molecule. We can see that there is one carbon atom present between two phenyl rings. Hence we can conclude that formaldehyde is used in the reaction. This trick can be used to answer a number of similar problems.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




