
What is the balanced net ionic equation for sodium hydroxide and acetylsalicylic acid?
Answer
527.7k+ views
Hint: A balanced net ionic equation is the chemical equation that involves only those ions, compounds, and elements that directly take part in the chemical reaction, and both mass and charge are balanced in such an equation. Acetylsalicylic acid and sodium hydroxide are an acid and base respectively. They react in a 1:1 ratio and produce salt and water.
Complete answer:
A chemical reaction can be represented in more than one type of equations. One of the methods of writing a chemical reaction is to write a net ionic equation. A net ionic equation includes only those species (element, molecule, ion, or compound) that are directly involved in the reaction. It is most frequently used to write acid-base neutralization reactions and redox reactions.
The ions or species that do not play any significant role in the reaction and are present in the reaction mixture both before and after the reaction is called a spectator ion.
Let us write the net balanced equation for the reaction between NaOH and acetylsalicylic acid.
Step 1 – Write a balanced chemical equation.
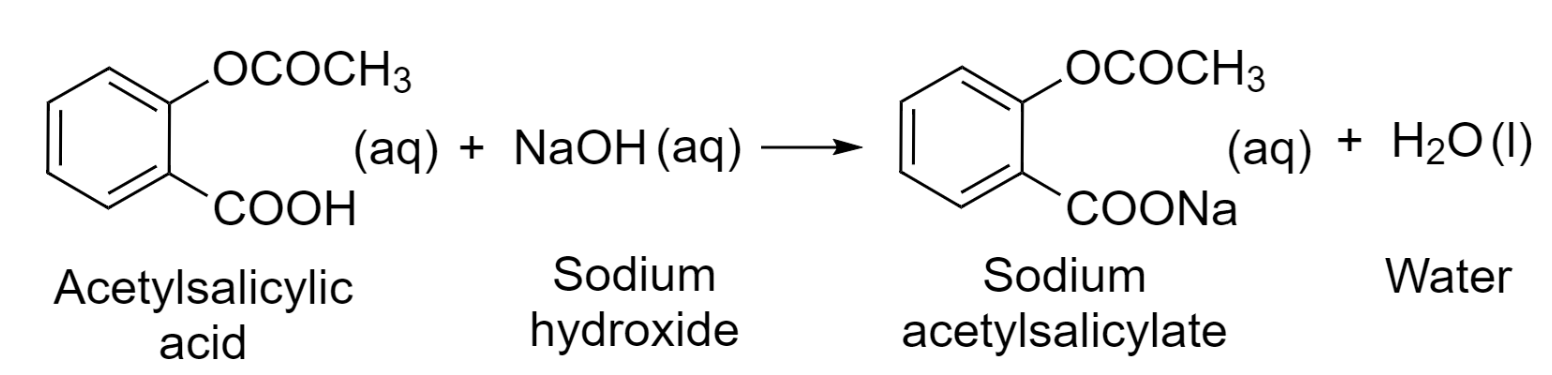
Step 2 – Write the equation in terms of ionic species by breaking down the electrolytes into ions they form in an aqueous solution. Species with (s), (l), or (g) states will remain unchanged.
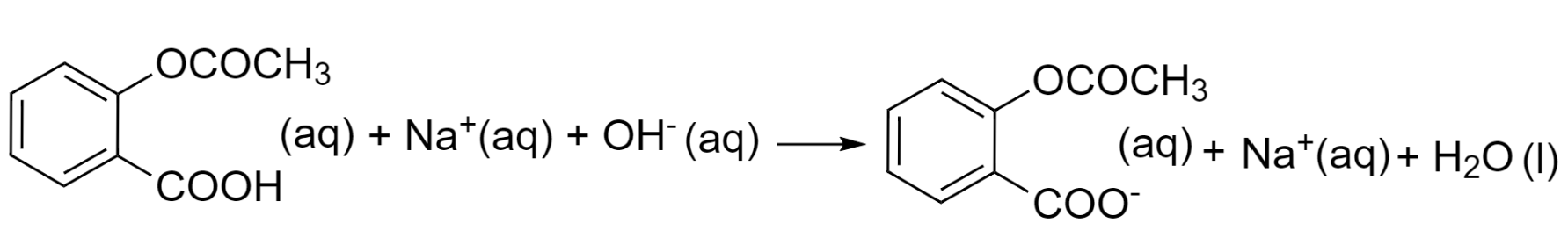
Step 3 – Look out for the spectator ion in an aqueous state. In this case, the sodium ion is a spectator ion as it is present on both sides of the equation. So, we will just cancel out sodium ions from both sides.
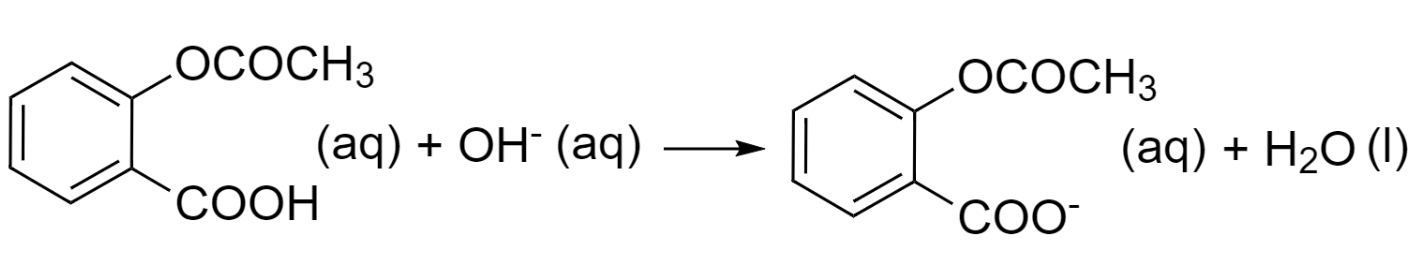
Thus, it is our required balanced net ionic equation.
Note:
While balancing an ionic equation, both the mass and charge should be equally balanced in the equation. The mass is balanced when there is an equal number of each element on both sides and the charge is said to be balanced when the overall charge is the same on the left-hand side and right-hand side of the equation.
Complete answer:
A chemical reaction can be represented in more than one type of equations. One of the methods of writing a chemical reaction is to write a net ionic equation. A net ionic equation includes only those species (element, molecule, ion, or compound) that are directly involved in the reaction. It is most frequently used to write acid-base neutralization reactions and redox reactions.
The ions or species that do not play any significant role in the reaction and are present in the reaction mixture both before and after the reaction is called a spectator ion.
Let us write the net balanced equation for the reaction between NaOH and acetylsalicylic acid.
Step 1 – Write a balanced chemical equation.

Step 2 – Write the equation in terms of ionic species by breaking down the electrolytes into ions they form in an aqueous solution. Species with (s), (l), or (g) states will remain unchanged.

Step 3 – Look out for the spectator ion in an aqueous state. In this case, the sodium ion is a spectator ion as it is present on both sides of the equation. So, we will just cancel out sodium ions from both sides.

Thus, it is our required balanced net ionic equation.
Note:
While balancing an ionic equation, both the mass and charge should be equally balanced in the equation. The mass is balanced when there is an equal number of each element on both sides and the charge is said to be balanced when the overall charge is the same on the left-hand side and right-hand side of the equation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




