
What is the basicity of $H_{3}PO_{3}$?
A.3
B.2
C.1
D.None of these
Answer
551.7k+ views
Hint: In order to solve the question, we need to know the dependency of the basicity of a compound. The base is a substance that can release $OH^{-}$ ions when dissolved in water. Similarly, an acid is. A substance that can release $H^{+}$ when dissolved in water. Not all bases and acids have the same ability to donate $OH^{-}$ ions. This ability defines the strength of a base and acid and basicity is a measure of this strength.
Complete answer:
- We must remember that basicity of an acid is the number of $H^{+}$ ions released, and the basicity of a base is the number of $OH^{-}$ ions released when dissolved in water.
- It determines the strength of an acid or base.
- The basicity of an acid depends on the number of ionizable hydrogen atoms present in the acid.
- The basicity of a base depends on the number of ionizable hydroxyl groups present in the base.
- $H_{3}PO_{3}$ is an acid since there are no hydroxyl groups present.
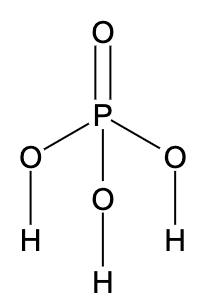
- As we can see in the skeleton structure, hydrogen atoms attached directly to the oxygen atoms are ionisable. The hydrogen atom attached directly to the phosphorus atom is reducing in nature and non-ionisable.
- Since there are two ionizable hydrogen groups in the given compound, the basicity of the acid is 2.
Therefore, the correct answer is (B).
Note:
It must be noted that the basicity of an acid with basicity 2 is also known as dibasic acid. The acids with a single ionisable hydrogen ion are known as monobasic acid and those with three ionisable hydrogen groups are known as tribasic acids.
Complete answer:
- We must remember that basicity of an acid is the number of $H^{+}$ ions released, and the basicity of a base is the number of $OH^{-}$ ions released when dissolved in water.
- It determines the strength of an acid or base.
- The basicity of an acid depends on the number of ionizable hydrogen atoms present in the acid.
- The basicity of a base depends on the number of ionizable hydroxyl groups present in the base.
- $H_{3}PO_{3}$ is an acid since there are no hydroxyl groups present.

- As we can see in the skeleton structure, hydrogen atoms attached directly to the oxygen atoms are ionisable. The hydrogen atom attached directly to the phosphorus atom is reducing in nature and non-ionisable.
- Since there are two ionizable hydrogen groups in the given compound, the basicity of the acid is 2.
Therefore, the correct answer is (B).
Note:
It must be noted that the basicity of an acid with basicity 2 is also known as dibasic acid. The acids with a single ionisable hydrogen ion are known as monobasic acid and those with three ionisable hydrogen groups are known as tribasic acids.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




