
\[BeCl_2\] is an electrophile and has a polymeric structure in solid state. Answer whether the above statement is true or false. If true enter 1, else enter 0.
Answer
581.1k+ views
Hint: First we should know the meaning of the terms electrophile and polymeric structure to get the appropriate answer. Here as we can see the molecule contain beryllium from s-block element, which have a tendency to donate electron and chlorine atom from p-block which have a tendency to accept electron to form \[BeCl_2\] molecule with covalent bond, have solid state.
Complete step by step answer:
> The meaning of electrophile is that the species that loves electrons is known as electrophile. In simple words, the atom which accepts electrons from another atom or molecule and forms a stable covalent bond is known as an electrophile.
> Now, we should also know the meaning of polymeric substances. The substance which is formed by the linkage of n number of monomers in branched, long chains, or cross chains or globular way is called polymeric structure. The organic molecule generally forms solid state polymeric structure because its monomer is having ionic character but when two monomers combine they combine via covalent bond as organic molecules combine to form a polymer.
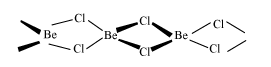
> Beryllium is surrounded by chlorine atoms in the given figure, the number of chlorine atoms are four out of which the two chlorine atoms is bounded to beryllium via covalent bond shown by normal line and other two chlorine atoms are bounded by coordinate bond which is shown by dark line. In the given figure, the beryllium atom has atomic number four and electronic configuration is it can donate two electrons. In chlorine the atomic number is seventeen and electronic configuration has a tendency to gain one electron hence two chlorine atoms will share beryllium’s two electrons to form the covalent bond.
> In \[BeCl_2\] molecule beryllium atom is electron deficient as it will require two more electrons from chlorine to complete its octet state, and, we know the electron loving species is called electrophile so, \[BeCl_2\] will act as electrophile and the structure as shown in the figure represents the structure of polymer with monomer as repeating unit. Here \[BeCl2\] will be a repeating unit,so it can form four bonds with four chlorine atoms. Hence \[BeCl2\] is an electrophile and has a polymeric structure in solid state.
Hence the answer is 1.
Note: We know that the electron deficient species is electrophile, here \[BeCl_2\] is the one which has a polymeric structure in solid state. \[BeCl_2\] is inorganic, colorless, hygroscopic solid which dissolves in the polar solvent and has similar properties to aluminum chloride. As beryllium has a diagonal relationship with aluminum. When two molecules of \[BeCl_2\] combines, chlorine atom surrounds the beryllium atom and forms tetrahedral structure as shown in the figure below, which is similar to that of the long chain polymer in which the monomer is \[BeCl_2\].
Complete step by step answer:
> The meaning of electrophile is that the species that loves electrons is known as electrophile. In simple words, the atom which accepts electrons from another atom or molecule and forms a stable covalent bond is known as an electrophile.
> Now, we should also know the meaning of polymeric substances. The substance which is formed by the linkage of n number of monomers in branched, long chains, or cross chains or globular way is called polymeric structure. The organic molecule generally forms solid state polymeric structure because its monomer is having ionic character but when two monomers combine they combine via covalent bond as organic molecules combine to form a polymer.
> Beryllium is surrounded by chlorine atoms in the given figure, the number of chlorine atoms are four out of which the two chlorine atoms is bounded to beryllium via covalent bond shown by normal line and other two chlorine atoms are bounded by coordinate bond which is shown by dark line. In the given figure, the beryllium atom has atomic number four and electronic configuration is it can donate two electrons. In chlorine the atomic number is seventeen and electronic configuration has a tendency to gain one electron hence two chlorine atoms will share beryllium’s two electrons to form the covalent bond.
> In \[BeCl_2\] molecule beryllium atom is electron deficient as it will require two more electrons from chlorine to complete its octet state, and, we know the electron loving species is called electrophile so, \[BeCl_2\] will act as electrophile and the structure as shown in the figure represents the structure of polymer with monomer as repeating unit. Here \[BeCl2\] will be a repeating unit,so it can form four bonds with four chlorine atoms. Hence \[BeCl2\] is an electrophile and has a polymeric structure in solid state.

Hence the answer is 1.
Note: We know that the electron deficient species is electrophile, here \[BeCl_2\] is the one which has a polymeric structure in solid state. \[BeCl_2\] is inorganic, colorless, hygroscopic solid which dissolves in the polar solvent and has similar properties to aluminum chloride. As beryllium has a diagonal relationship with aluminum. When two molecules of \[BeCl_2\] combines, chlorine atom surrounds the beryllium atom and forms tetrahedral structure as shown in the figure below, which is similar to that of the long chain polymer in which the monomer is \[BeCl_2\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




