
Benzene diazonium chloride on reaction with phenol in a basic medium gives:
A) diphenyl ether
B) p-Hydroxy azobenzene
C) chlorobenzene
D) benzene
Answer
594.3k+ views
Hint: When the concentration of OH- ion in solution is more, it is basic in nature. We call such a solution a basic medium.
Complete step by step answer:
Benzene diazonium chloride in reaction with phenol in a basic medium gives p-Hydroxy azobenzene.
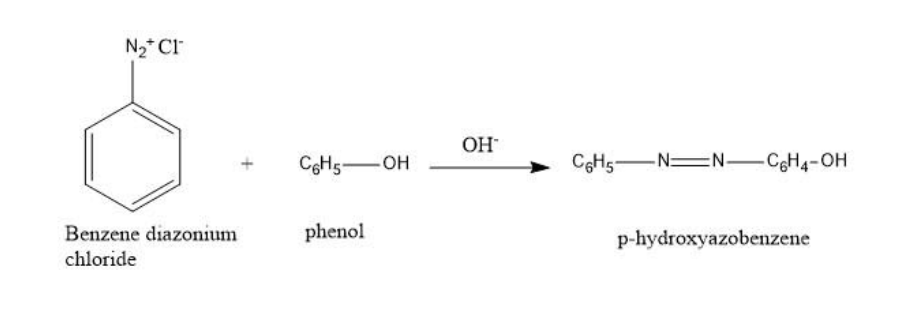
So, out of the given options, B is the correct option, that is, p-Hydroxy azobenzene.
Additional information:
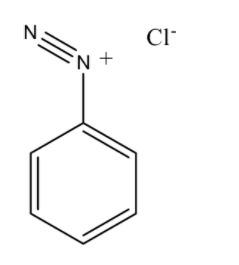
The structure of Benzene diazonium chloride is
And the structure of p-Hydroxy azobenzene is,
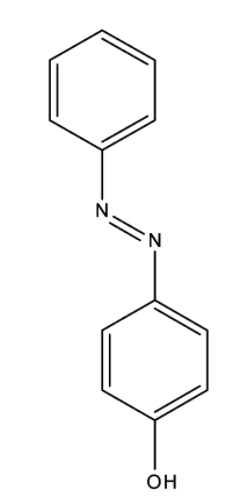
Benzene diazonium chloride has the chemical formula ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}{\rm{Cl}}{{\rm{N}}_{\rm{2}}}$. Benzene diazonium chloride is a salt of a chloride and diazonium cation. Benzene diazonium chloride exists as a colourless solid and it is soluble in polar solvents such as in water. It acts as the parent member of the aryl diazonium compounds. This compound is widely used in organic chemistry. As this salt is unstable, it is not available commercially but is prepared according to the demand. p-hydroxyazobenzene has chemical formula ${\rm{C}}{{\rm{l}}_{\rm{2}}}{{\rm{H}}_{{\rm{10}}}}{{\rm{N}}_{{\rm{20}}}}$ and has the atomic number 198.221. It has a density of $1.13{\rm{g/c}}{{\rm{m}}^{\rm{3}}}$, and has a boiling point of ${369.812^o}C$ and has a refractive index of 1.596.
The mechanism for the reaction of benzene diazonium chloride on reaction with phenol can be given as follows:
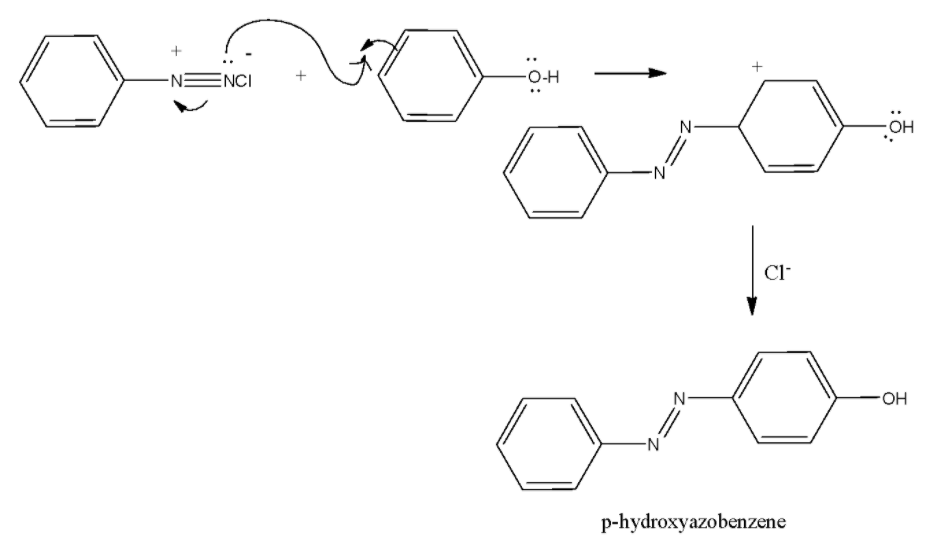
Note:
Benzene diazonium chloride has the chemical formula ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}{\rm{Cl}}{{\rm{N}}_{\rm{2}}}$. This compound is soluble in polar solvent and it exists as a colourless solid.
Complete step by step answer:
Benzene diazonium chloride in reaction with phenol in a basic medium gives p-Hydroxy azobenzene.

So, out of the given options, B is the correct option, that is, p-Hydroxy azobenzene.
Additional information:
The structure of Benzene diazonium chloride is

And the structure of p-Hydroxy azobenzene is,

Benzene diazonium chloride has the chemical formula ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}{\rm{Cl}}{{\rm{N}}_{\rm{2}}}$. Benzene diazonium chloride is a salt of a chloride and diazonium cation. Benzene diazonium chloride exists as a colourless solid and it is soluble in polar solvents such as in water. It acts as the parent member of the aryl diazonium compounds. This compound is widely used in organic chemistry. As this salt is unstable, it is not available commercially but is prepared according to the demand. p-hydroxyazobenzene has chemical formula ${\rm{C}}{{\rm{l}}_{\rm{2}}}{{\rm{H}}_{{\rm{10}}}}{{\rm{N}}_{{\rm{20}}}}$ and has the atomic number 198.221. It has a density of $1.13{\rm{g/c}}{{\rm{m}}^{\rm{3}}}$, and has a boiling point of ${369.812^o}C$ and has a refractive index of 1.596.
The mechanism for the reaction of benzene diazonium chloride on reaction with phenol can be given as follows:

Note:
Benzene diazonium chloride has the chemical formula ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}{\rm{Cl}}{{\rm{N}}_{\rm{2}}}$. This compound is soluble in polar solvent and it exists as a colourless solid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




