
Benzene reacts with chlorine in presence of light to yield:
A.chlorobenzene
B.trichlorobenzene
C.tetrachlorobenzene
D.benzene hexachloride
Answer
585.9k+ views
Hint:When an addition reaction involves initially the attack by an electrophile, the reaction is referred to as electrophilic addition. Compounds containing carbon-carbon double bond and triple bond undergo such reactions.
Complete step by step answer:
We know that the reaction of benzene with chlorine in the presence of light is an electrophilic addition reaction. The reaction of benzene with chlorine in presence of light gives results in the generation of benzene hexachloride. Benzene hexachloride is also called Gammexane. IUPAC name of BHC is 1,2,3,4,5,6-Hexachlorocyclohexane. We can write the reaction of it as follows:
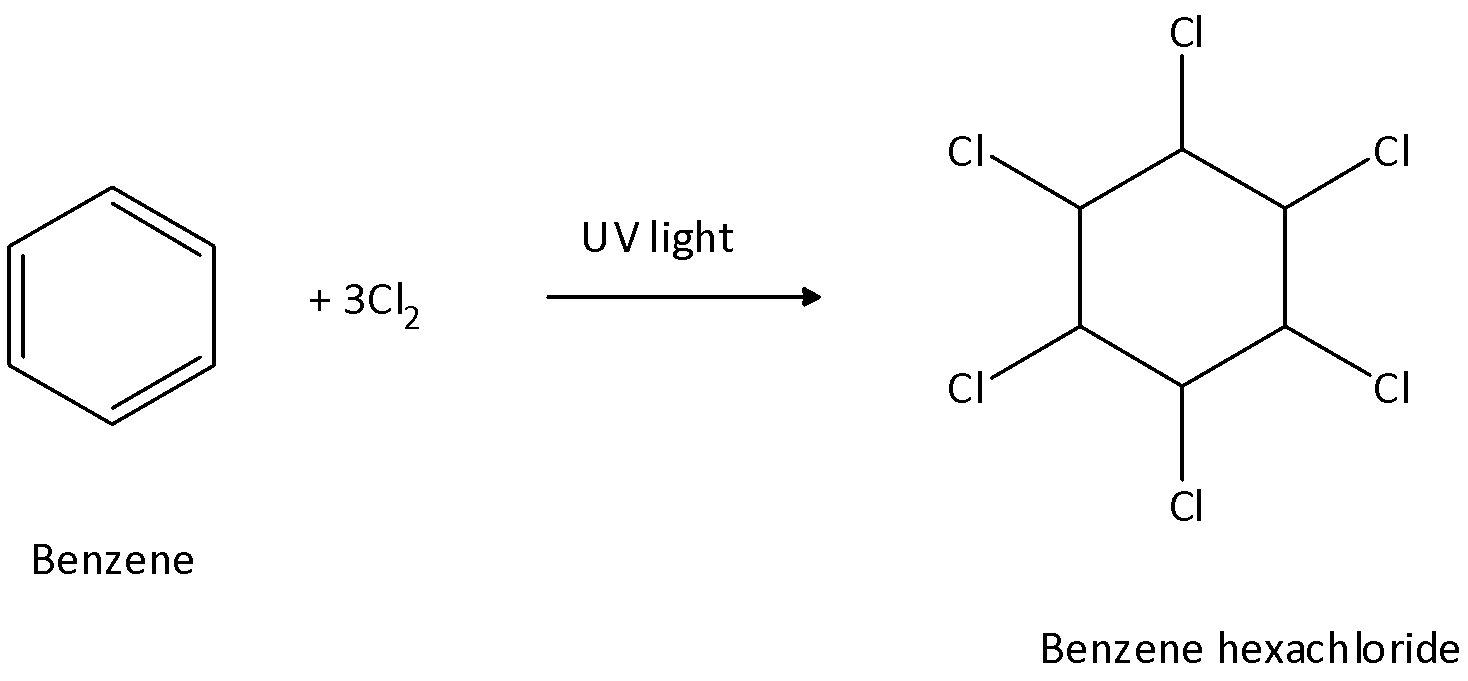
Therefore we can say that out of the given four options, D is the correct option.
Additional information:
We know that a cyclic compound is a compound where atoms are connected or joined in the form of a ring structure. In cyclic compounds, all the connected atoms can be carbon, that is carbocycles, none of the atoms can be carbon which are referred to as inorganic cyclic compounds and atoms can be both carbon and non-carbon which are referred to as heterocyclic compounds. Benzene is considered to be the most stable cyclic compound.
Note:
Benzene is an aromatic carbon with 6 carbon atoms. Benzene has the chemical formula \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}\].This compound is soluble in water solution and it is a colourless compound. Benzene-hexachloride has the chemical formula \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{C}}{{\text{l}}_{\text{6}}}\]. This compound is soluble in solvents like chloroform.
Complete step by step answer:
We know that the reaction of benzene with chlorine in the presence of light is an electrophilic addition reaction. The reaction of benzene with chlorine in presence of light gives results in the generation of benzene hexachloride. Benzene hexachloride is also called Gammexane. IUPAC name of BHC is 1,2,3,4,5,6-Hexachlorocyclohexane. We can write the reaction of it as follows:

Therefore we can say that out of the given four options, D is the correct option.
Additional information:
We know that a cyclic compound is a compound where atoms are connected or joined in the form of a ring structure. In cyclic compounds, all the connected atoms can be carbon, that is carbocycles, none of the atoms can be carbon which are referred to as inorganic cyclic compounds and atoms can be both carbon and non-carbon which are referred to as heterocyclic compounds. Benzene is considered to be the most stable cyclic compound.
Note:
Benzene is an aromatic carbon with 6 carbon atoms. Benzene has the chemical formula \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}\].This compound is soluble in water solution and it is a colourless compound. Benzene-hexachloride has the chemical formula \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{C}}{{\text{l}}_{\text{6}}}\]. This compound is soluble in solvents like chloroform.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




