
How many benzene rings are present in Naphthalene?
(A) $2$
(B) $3$
(C) $4$
(D) $5$
Answer
565.5k+ views
Hint:We all know about Naphthalene. It is obtained from crude oil or coal tar. It is also used as a fumigant in households. It is the simplest polycyclic aromatic hydrocarbon. Also, it has the molecular formula ${C_{10}}{H_8}$. Like benzene, it is a planar molecule but the bond lengths between atoms in naphthalene are different.
Complete answer:We all know about Naphthalene. It is generally used as a fumigant in households. Fumigants are those substances that are used to kill insects, nematodes, etc by turning themselves from solid into toxic vapors. These substances are poisonous and toxic. Naphthalene is an organic compound, with a molecular formula ${C_{10}}{H_8}$ . It is a polycyclic aromatic hydrocarbon with two fused benzene rings. It is a white crystalline solid. It has the following structure:
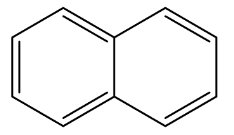
We know that bond length is the average distance between the nuclei of two bonded atoms. Bond length is a function of hybridization. The bond lengths between carbon-carbon atoms are not the same in Naphthalene. As naphthalene is a polycyclic aromatic hydrocarbon, it consists of two fused benzene rings, therefore there are alternate double bonds and single bonds. Therefore the hybridization changes and thus the bond lengths changes. Resonance also occurs in the naphthalene structure.
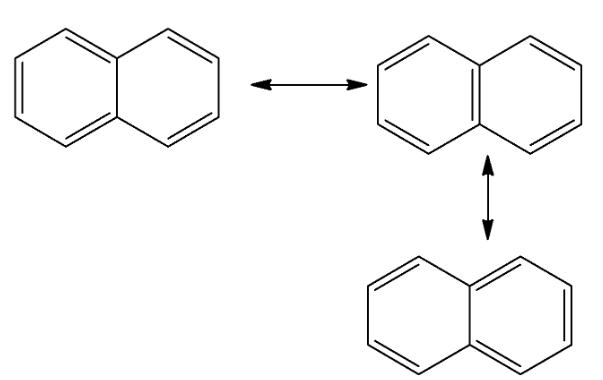
Resonance is a property in which an aromatic compound can be represented by two or more resonating structures. These structures have the same number of electrons but differ in the arrangement of electrons. Resonance occurs due to delocalized pi-electrons. The double-headed arrows used in resonating structures represent that the actual structure is the average of the resonance structures.
Thus, the correct option is $A$.
Note:It is important to note that Naphthalene is the simplest polycyclic aromatic hydrocarbon. According to Huckel’s rule for a molecule to be aromatic, it should be planar, cyclic, and must have $(4n + 2)$ pi electrons. Naphthalene is a planar, cyclic molecule with $10\left[ {4 \times 2 + 2} \right]$ pi electrons. Thus Naphthalene is an aromatic molecule.
Complete answer:We all know about Naphthalene. It is generally used as a fumigant in households. Fumigants are those substances that are used to kill insects, nematodes, etc by turning themselves from solid into toxic vapors. These substances are poisonous and toxic. Naphthalene is an organic compound, with a molecular formula ${C_{10}}{H_8}$ . It is a polycyclic aromatic hydrocarbon with two fused benzene rings. It is a white crystalline solid. It has the following structure:

We know that bond length is the average distance between the nuclei of two bonded atoms. Bond length is a function of hybridization. The bond lengths between carbon-carbon atoms are not the same in Naphthalene. As naphthalene is a polycyclic aromatic hydrocarbon, it consists of two fused benzene rings, therefore there are alternate double bonds and single bonds. Therefore the hybridization changes and thus the bond lengths changes. Resonance also occurs in the naphthalene structure.

Resonance is a property in which an aromatic compound can be represented by two or more resonating structures. These structures have the same number of electrons but differ in the arrangement of electrons. Resonance occurs due to delocalized pi-electrons. The double-headed arrows used in resonating structures represent that the actual structure is the average of the resonance structures.
Thus, the correct option is $A$.
Note:It is important to note that Naphthalene is the simplest polycyclic aromatic hydrocarbon. According to Huckel’s rule for a molecule to be aromatic, it should be planar, cyclic, and must have $(4n + 2)$ pi electrons. Naphthalene is a planar, cyclic molecule with $10\left[ {4 \times 2 + 2} \right]$ pi electrons. Thus Naphthalene is an aromatic molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




