
Benzoic acid is treated with $ SOC{l_2} $ and the product X is formed with ammonia to give Y. Y on reaction with $ B{r_2} $ and $ KOH $ gives Z. What is Z in the reaction?
Answer
494.4k+ views
Hint: Benzoic acid is a carboxylic acid when treated with thionyl chloride $ \left( {SOC{l_2}} \right) $ the carboxylic acid group changes to acid chloride. When this acid chloride with ammonia forms an amide. An amide when treated with $ B{r_2} $ and $ KOH $ undergoes Hoffmann reaction to form a primary amine. Thus, the final product is aniline.
Complete Step By Step Answer:
Chemical compounds are classified based on the functional group present in the compounds. Reagents added to the compounds lead to the formation of new compounds.
Benzoic acid is a carboxylic acid with a $ - COOH $ group on a benzene ring. Thionyl chloride is a chlorinating agent with a molecular formula of $ SOC{l_2} $ . When Benzoic acid is treated with $ SOC{l_2} $ the carboxylic acid group converts into $ - COCl $ . When the newly formed compound is treated with ammonia the acid chloride group converts into an amide group. As the chlorine is replaced by an amine group. When this benzamide is treated with $ B{r_2} $ and $ KOH $ undergoes Hoffmann bromination and forms an aniline. In this reaction an amide group converts into an amine group.
The chemical reaction is:
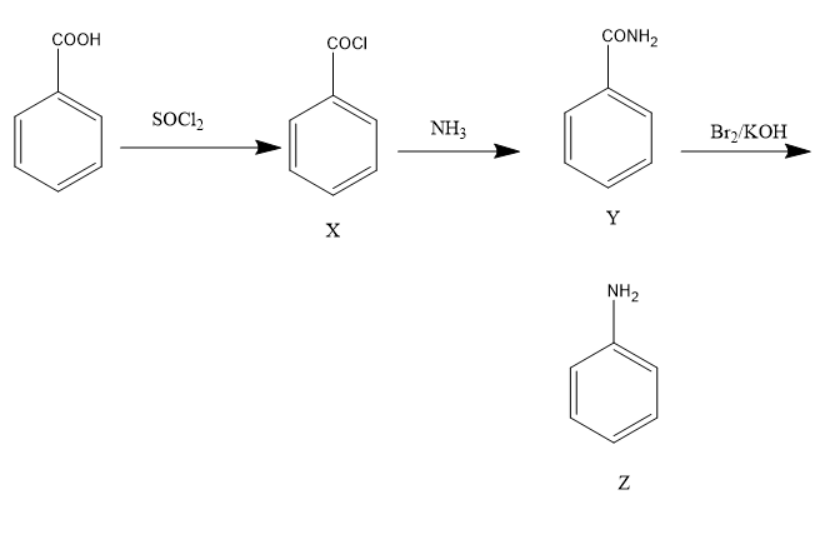
Thus, the product Z is aniline.
Note:
Substitution reaction is the reaction in which one group can be replaced by another compound. In the above reaction, thionyl chloride is a chlorinating reagent that replaces hydroxyl group by chlorine. Chlorine is replaced by amine by the action of ammonia. Amide can be replaced by an amine by bromine and potassium hydroxide.
Complete Step By Step Answer:
Chemical compounds are classified based on the functional group present in the compounds. Reagents added to the compounds lead to the formation of new compounds.
Benzoic acid is a carboxylic acid with a $ - COOH $ group on a benzene ring. Thionyl chloride is a chlorinating agent with a molecular formula of $ SOC{l_2} $ . When Benzoic acid is treated with $ SOC{l_2} $ the carboxylic acid group converts into $ - COCl $ . When the newly formed compound is treated with ammonia the acid chloride group converts into an amide group. As the chlorine is replaced by an amine group. When this benzamide is treated with $ B{r_2} $ and $ KOH $ undergoes Hoffmann bromination and forms an aniline. In this reaction an amide group converts into an amine group.
The chemical reaction is:

Thus, the product Z is aniline.
Note:
Substitution reaction is the reaction in which one group can be replaced by another compound. In the above reaction, thionyl chloride is a chlorinating reagent that replaces hydroxyl group by chlorine. Chlorine is replaced by amine by the action of ammonia. Amide can be replaced by an amine by bromine and potassium hydroxide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




