
Benzoin is:
A. Compound containing an aldehyde and a ketonic group
B. $\alpha ,\beta - $unsaturated acid
C. $\alpha - $hydroxy aldehyde
D. $\alpha - $hydroxy ketone
Answer
573.6k+ views
Hint: The formula of benzoin is ${\text{PhCH(OH)COPh}}$. The benzoin contains –OH group attached with alpha carbon of–CO functional group and two phenyl rings. One phenyl ring attaches with –CO functional group and the second attaches with alpha carbon.
Complete Step by step answer: The structure of benzoin is as follows:
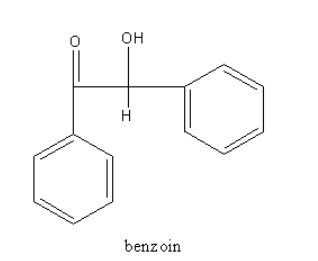
Benzoin has a keto functional group, so it is a ketone. The structure of the keto functional group is shown as follows:
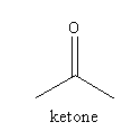
The carbon directly attached to the carbon of the keto functional group is known as alpha carbon. The alpha carbon in simple ketone and benzoin is shown as follows:
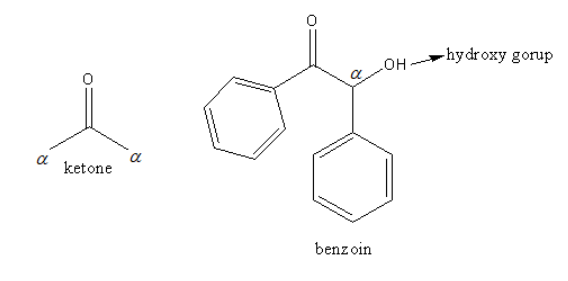
The benzoin has a hydroxy and one phenyl ring at the alpha position. The second phenyl has attached another side of the ketone.
So, benzoin is an $\alpha - $hydroxy ketone.
Therefore, option (D) $\alpha - $hydroxy ketone is correct.
Additional information: Benzoin is formed by the condensation reaction of two benzaldehydes. The reaction is known as the benzoin condensation reaction. The reagent is used in benzoin condensation sodium cyanide. The reaction initiates with a nucleophile such as cyanide which attacks carbonyl of one aldehyde and forms a nucleophile. The nucleophilic aldehyde attacks the second aldehyde. Then the elimination of cyanide and proton transfer gives the product benzoin.
Note: Benzoin is a chiral molecule. It exists as enantiomers R and S. Benzoin has a camphor-like odour and appears as off-white crystal. Benzoin is an alpha-hydroxy ketone. Ketone having a hydroxyl group at beta-carbon is known as beta-hydroxy ketone.
Complete Step by step answer: The structure of benzoin is as follows:

Benzoin has a keto functional group, so it is a ketone. The structure of the keto functional group is shown as follows:

The carbon directly attached to the carbon of the keto functional group is known as alpha carbon. The alpha carbon in simple ketone and benzoin is shown as follows:

The benzoin has a hydroxy and one phenyl ring at the alpha position. The second phenyl has attached another side of the ketone.
So, benzoin is an $\alpha - $hydroxy ketone.
Therefore, option (D) $\alpha - $hydroxy ketone is correct.
Additional information: Benzoin is formed by the condensation reaction of two benzaldehydes. The reaction is known as the benzoin condensation reaction. The reagent is used in benzoin condensation sodium cyanide. The reaction initiates with a nucleophile such as cyanide which attacks carbonyl of one aldehyde and forms a nucleophile. The nucleophilic aldehyde attacks the second aldehyde. Then the elimination of cyanide and proton transfer gives the product benzoin.
Note: Benzoin is a chiral molecule. It exists as enantiomers R and S. Benzoin has a camphor-like odour and appears as off-white crystal. Benzoin is an alpha-hydroxy ketone. Ketone having a hydroxyl group at beta-carbon is known as beta-hydroxy ketone.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




