
Benzyl chloride is converted to _________ using sodium hydroxide solution.
a) Phenol
b) o-chloromethyl phenol
c) p-chloromethyl phenol
d) benzyl alcohol
Answer
597k+ views
Hint: For identifying the end product of a reaction, look at the reagent provided in the question. Sodium hydroxide solution or aqueous NaOH is a strong base. Therefore, this reaction will result in a substitution product.
Complete step by step answer:
The data provided to us in the question is –
Benzyl chloride – reactant. It is a benzyl halide, in which the halogen acts as a leaving group.
Nucleophile – Sodium hydroxide solution or aqueous NaOH. It is a very good base and a strong nucleophile
In organic chemistry, we can predict the product for a chemical reaction by looking at the reaction compound and the reagent. Since we have a benzylic halide with a strong nucleophile, the reaction will proceed via SN2 mechanism and a substitution product will be formed.
Therefore, the hydroxyl group replaces the chloride group.
The reaction can be represented as –
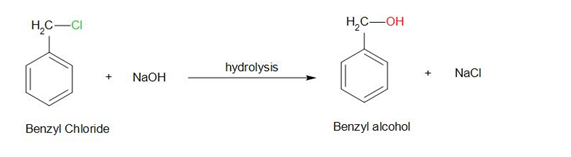
Therefore, the answer is – option (d) – Benzyl Alcohol.
Additional Information:
Benzylic halides are the compounds in which the halogen is bonded to a \[s{{p}^{3}}\] hybridized carbon atom next to an aromatic ring.
Note: There are two types of substitution reactions –
1) Substitution nucleophilic unimolecular
-It is also known as SN1 type reaction, because it is a first order reaction.
-It takes place in a polar protic solution.
-A weak nucleophile is needed in such reactions.
2) Substitution nucleophilic bimolecular
-It is also known as SN2 type reaction, because it is a second order reaction.
-It takes place in a polar aprotic solution.
-A strong nucleophile is needed in such reactions.
Complete step by step answer:
The data provided to us in the question is –
Benzyl chloride – reactant. It is a benzyl halide, in which the halogen acts as a leaving group.
Nucleophile – Sodium hydroxide solution or aqueous NaOH. It is a very good base and a strong nucleophile
In organic chemistry, we can predict the product for a chemical reaction by looking at the reaction compound and the reagent. Since we have a benzylic halide with a strong nucleophile, the reaction will proceed via SN2 mechanism and a substitution product will be formed.
Therefore, the hydroxyl group replaces the chloride group.
The reaction can be represented as –

Therefore, the answer is – option (d) – Benzyl Alcohol.
Additional Information:
Benzylic halides are the compounds in which the halogen is bonded to a \[s{{p}^{3}}\] hybridized carbon atom next to an aromatic ring.
Note: There are two types of substitution reactions –
1) Substitution nucleophilic unimolecular
-It is also known as SN1 type reaction, because it is a first order reaction.
-It takes place in a polar protic solution.
-A weak nucleophile is needed in such reactions.
2) Substitution nucleophilic bimolecular
-It is also known as SN2 type reaction, because it is a second order reaction.
-It takes place in a polar aprotic solution.
-A strong nucleophile is needed in such reactions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




