
Beryllium shows diagonal relationship with:
A. B
B. Mg
C. Al
D. Na
Answer
592.2k+ views
Hint: Diagonal relationship is a property that occurs between certain pairs of the elements present diagonally in the second period and third period in the periodic table. These pairs of elements show similar properties. For example, boron and silicon both are semiconductors in nature.
Complete step by step answer:
- We know that periodic contains four blocks, s, p d, and f blocks.
- In those blocks only few elements show the property of diagonal relationship.
- The below image shows which elements show the diagonal relationship.
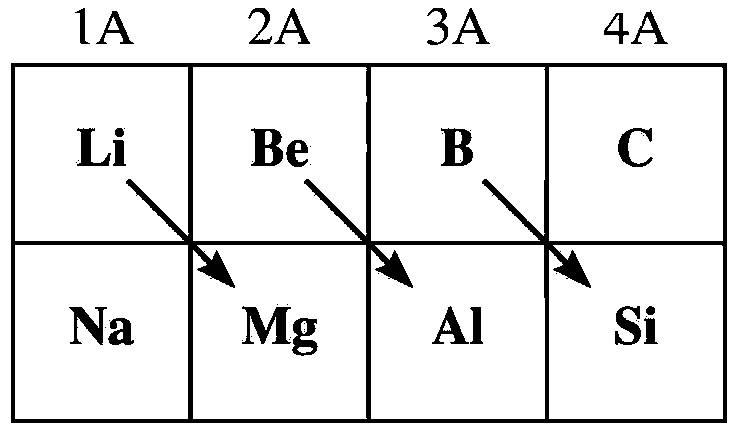
- From the above image we can say that lithium and magnesium show diagonal relationship.
- In the question it is asked that Beryllium shows diagonal relationship with which metal.
- By seeing the above image we can easily say that beryllium is in diagonal relationship with aluminum.
So, the correct option is C.
Additional information:
Both the metals (beryllium and aluminum) have an affinity to form covalent compounds. For example the chlorides of both the elements being covalent and are soluble in organic solvents.
Beryllium and aluminum have the same electronegativity and the polarizing power ratio of their ions is similar.
Both \[BeC{{l}_{2}}\] and \[AlC{{l}_{3}}\] act as Lewis acids.
Both the metals dissolve in strong alkalies (like NaOH) to form soluble complexes.
The oxides of both the metals Be (BeO) and Al (\[A{{l}_{2}}{{O}_{3}}\]) are hard, high melting point and are insoluble solids.
Carbides of Beryllium and aluminum metals react with water and liberate methane gas a product.
Note: Beryllium belongs to the 2A (s block element) group and Aluminum belongs to the 3A (p block element) group. The diagonal relation is not going to depend on which block they belong to. It is going to depend on the properties that the elements exhibit.
Complete step by step answer:
- We know that periodic contains four blocks, s, p d, and f blocks.
- In those blocks only few elements show the property of diagonal relationship.
- The below image shows which elements show the diagonal relationship.

- From the above image we can say that lithium and magnesium show diagonal relationship.
- In the question it is asked that Beryllium shows diagonal relationship with which metal.
- By seeing the above image we can easily say that beryllium is in diagonal relationship with aluminum.
So, the correct option is C.
Additional information:
Both the metals (beryllium and aluminum) have an affinity to form covalent compounds. For example the chlorides of both the elements being covalent and are soluble in organic solvents.
Beryllium and aluminum have the same electronegativity and the polarizing power ratio of their ions is similar.
Both \[BeC{{l}_{2}}\] and \[AlC{{l}_{3}}\] act as Lewis acids.
Both the metals dissolve in strong alkalies (like NaOH) to form soluble complexes.
The oxides of both the metals Be (BeO) and Al (\[A{{l}_{2}}{{O}_{3}}\]) are hard, high melting point and are insoluble solids.
Carbides of Beryllium and aluminum metals react with water and liberate methane gas a product.
Note: Beryllium belongs to the 2A (s block element) group and Aluminum belongs to the 3A (p block element) group. The diagonal relation is not going to depend on which block they belong to. It is going to depend on the properties that the elements exhibit.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




