
${ BF }_{ 3 }$ and ${ NH }_{ 3 }$ undergo Lewis acid-base reaction forming an adduct. Which picture below currently represents the curved arrow notation for the initial Lewis acid-base interaction in this reaction, what is the Lewis acid and the Lewis base?
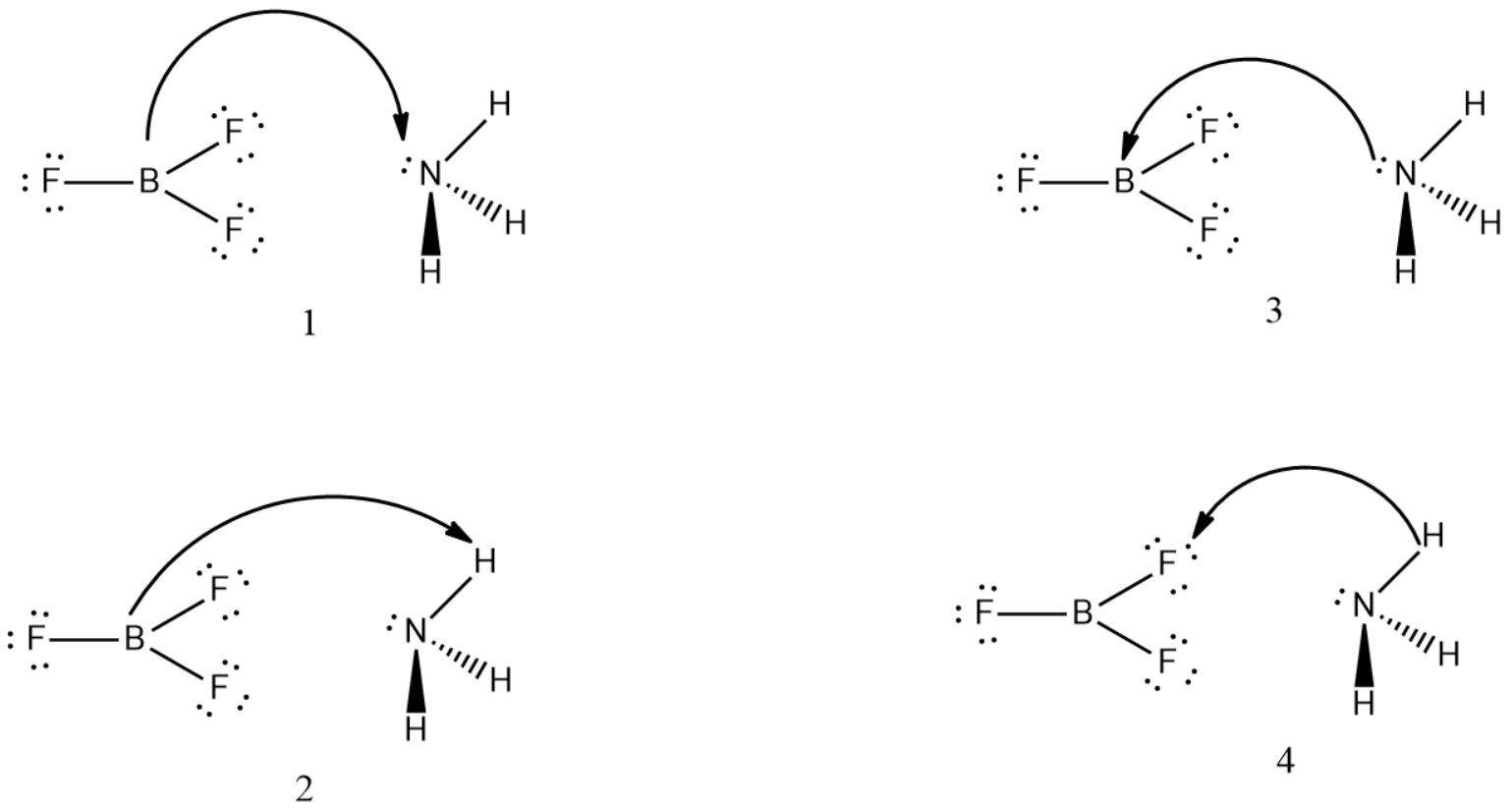
(A). Picture (1) is correct; ${ NH }_{ 3 }$ is the Lewis acid and ${ BF }_{ 3 }$ is the Lewis base.
(B). Picture (2) is correct; ${ BF }_{ 3 }$ is the Lewis acid and ${ NH }_{ 3 }$ is the Lewis base.
(C). Picture (3) is correct; ${ NH }_{ 3 }$ is the Lewis acid and ${ BF }_{ 3 }$ is the Lewis base.
(D). Picture (4) is correct; ${ BF }_{ 3 }$ is the Lewis acid and ${ NH }_{ 3 }$ is the Lewis base.

Answer
596.4k+ views
- Hint: A Lewis base will donate a pair of electrons while a Lewis acid will accept a pair of electrons. In this case, the electrons will be accepted by the one who has a vacant orbital.
Complete step-by-step solution -
The interaction between is a Lewis acid-base interaction, in which one of them donates a pair of electrons to the other. According to Lewis acid-base theory, the Lewis acid will accept a pair of electrons and a Lewis base will donate a pair of electrons.
The electronic configuration of Boron (B) is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 1 }$. As we can see there are three electrons in valence orbitals 2s and 2p.
A valence orbital is the outermost orbital from which the electron is gained or lost in case of ionic bond formation or from which the electron is shared in case of covalent bond formation.
Boron has 3 electrons in valence orbitals, so it forms 3 bonds with fluorine in ${ BF }_{ 3 }$. As we know, in the p orbital there are 3 spaces where 2 electrons can be accommodated in each of them giving a total capacity of 6 electrons in p orbital. In the case of Boron, there is just 1 electron in it so p orbital is vacant.
A vacant p orbital can accept electrons and thus boron having a vacant p orbital can accept electrons. So, it is a Lewis acid.
The electronic configuration of Nitrogen (N) is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 3 }$. As we can see there are three electrons in the 2p orbital. So, the orbital is occupied in all its three spaces.
2p Orbital having 3 electrons. Therefore, it cannot accept any more electrons.
The 2s orbital is filled and has a pair of electrons. So, that is the pair of electrons that nitrogen can donate. So, in this case, ${ NH }_{ 3 }$ will donate the electrons to the vacant orbitals of ${ BF }_{ 3 }$. So, ${ NH }_{ 3 }$ is the Lewis base and ${ BF }_{ 3 }$ is the Lewis acid for this reaction.
In this case, Picture 2 represents this scenario correctly, and therefore, option B is the correct one.
Note: Having the vacant orbital is the main key to this problem. Both boron and nitrogen form 3 bonds with 3 fluorine atoms and 3 hydrogen atoms respectively. But only one of them has a vacant orbital to accommodate the incoming electrons. Don’t confuse between them.
Complete step-by-step solution -
The interaction between is a Lewis acid-base interaction, in which one of them donates a pair of electrons to the other. According to Lewis acid-base theory, the Lewis acid will accept a pair of electrons and a Lewis base will donate a pair of electrons.
The electronic configuration of Boron (B) is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 1 }$. As we can see there are three electrons in valence orbitals 2s and 2p.
A valence orbital is the outermost orbital from which the electron is gained or lost in case of ionic bond formation or from which the electron is shared in case of covalent bond formation.
Boron has 3 electrons in valence orbitals, so it forms 3 bonds with fluorine in ${ BF }_{ 3 }$. As we know, in the p orbital there are 3 spaces where 2 electrons can be accommodated in each of them giving a total capacity of 6 electrons in p orbital. In the case of Boron, there is just 1 electron in it so p orbital is vacant.
A vacant p orbital can accept electrons and thus boron having a vacant p orbital can accept electrons. So, it is a Lewis acid.
The electronic configuration of Nitrogen (N) is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 3 }$. As we can see there are three electrons in the 2p orbital. So, the orbital is occupied in all its three spaces.
2p Orbital having 3 electrons. Therefore, it cannot accept any more electrons.
The 2s orbital is filled and has a pair of electrons. So, that is the pair of electrons that nitrogen can donate. So, in this case, ${ NH }_{ 3 }$ will donate the electrons to the vacant orbitals of ${ BF }_{ 3 }$. So, ${ NH }_{ 3 }$ is the Lewis base and ${ BF }_{ 3 }$ is the Lewis acid for this reaction.
In this case, Picture 2 represents this scenario correctly, and therefore, option B is the correct one.
Note: Having the vacant orbital is the main key to this problem. Both boron and nitrogen form 3 bonds with 3 fluorine atoms and 3 hydrogen atoms respectively. But only one of them has a vacant orbital to accommodate the incoming electrons. Don’t confuse between them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




