
Bombardment of aluminum by $\alpha $-particle leads to disintegration in two ways, (i) and (ii) as shown in the figure. Products X, Y and Z respectively are:
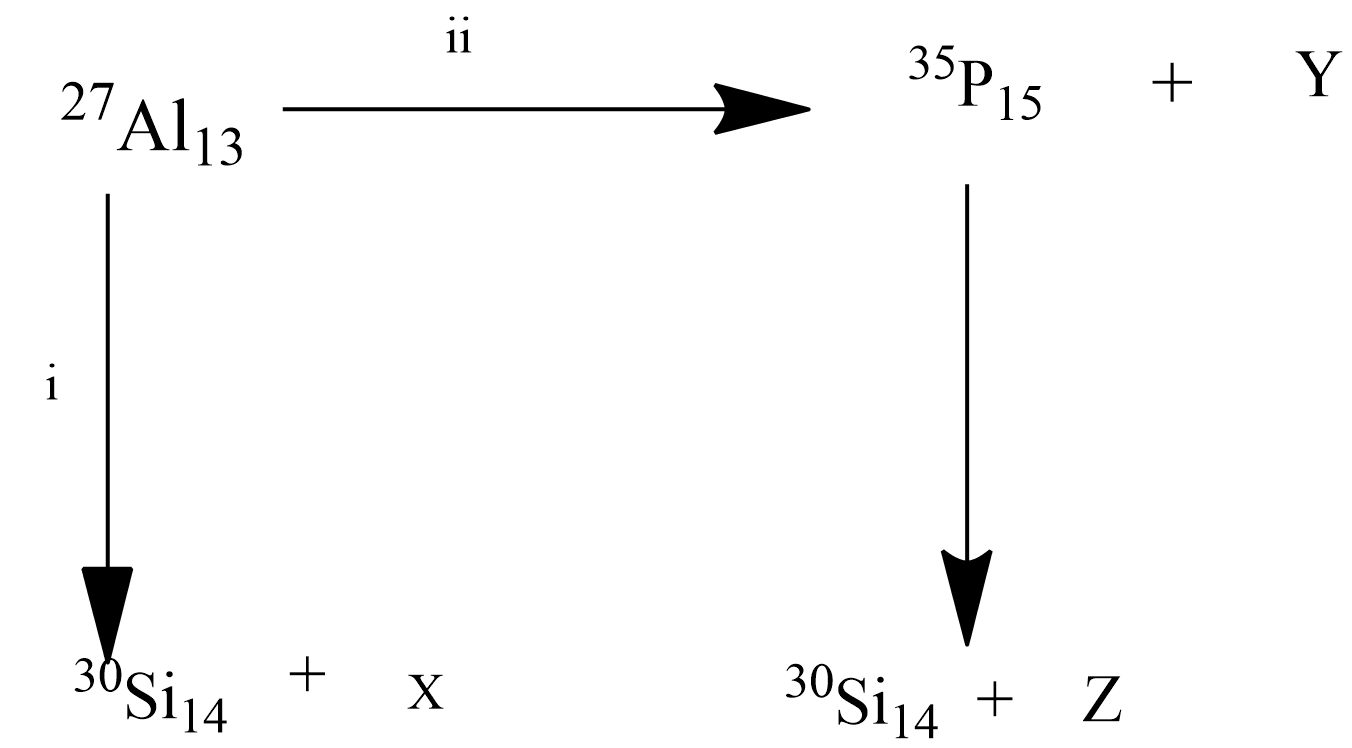
(A)proton, neutron, positron
(B)neutron, positron, proton
(C)proton, positron, neutron
(D)positron, proton, neutron
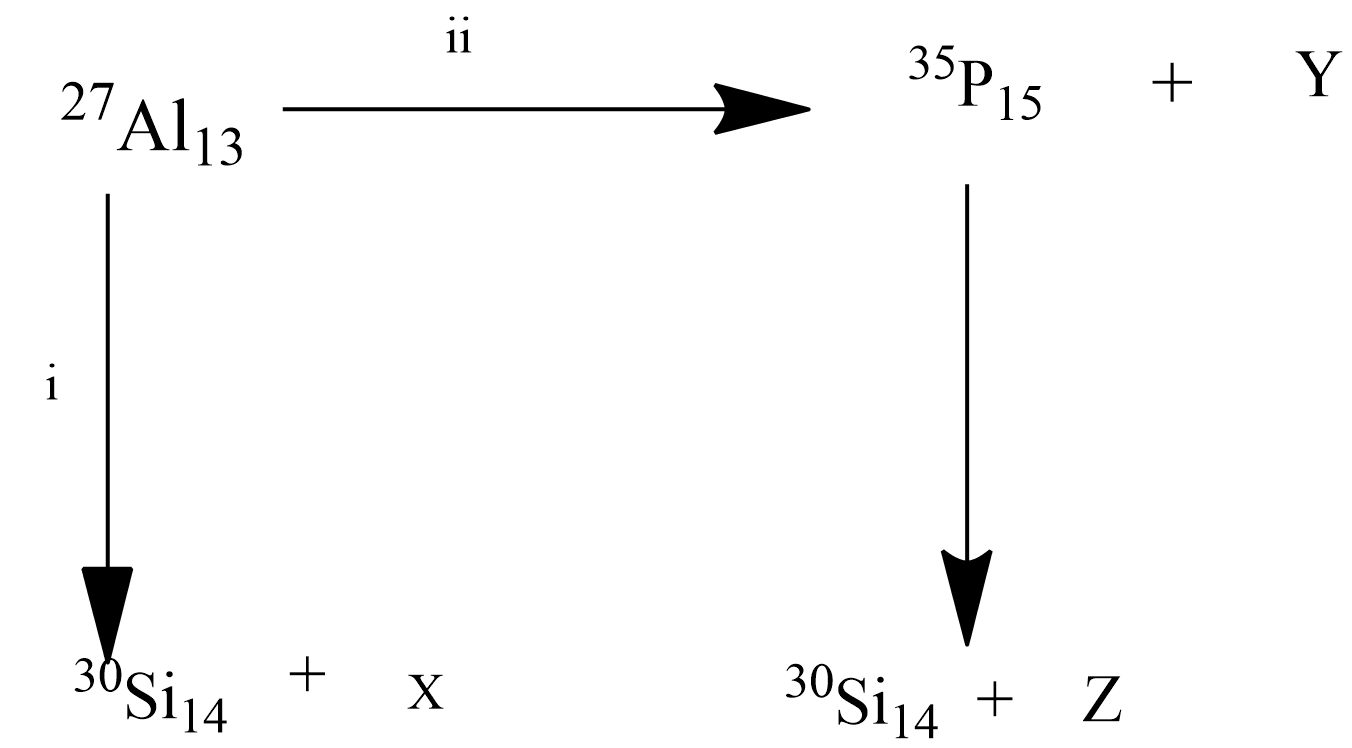
Answer
579.6k+ views
Hint: Alpha $\left( \alpha \right)$-particle is positive charged fast moving particle with two protons and two neutrons $_{\text{2}}^{\text{4}}{\text{He}}$. The products formed upon addition of alpha particles to any element can be known from the total mass number (A) and atomic number (Z).
Complete step by step solution:
Given that aluminum gets disintegrated in two ways by the addition of an alpha particle.
In one way $_{{\text{13}}}^{{\text{27}}}{\text{Al}}$ gives silicon ($_{{\text{14}}}^{{\text{30}}}{\text{Si}}$) and some other species marked as ‘x’ and in the second way it forms phosphorus $\left( {_{15}^{35}{\text{P}}} \right)$ and a species ‘y’ in the first case and the phosphorus in turn forms silicon-14 isotope $\left( {_{{\text{14}}}^{{\text{30}}}{\text{Si}}} \right)$.
So let us find out the unknown species ‘x’. The chemical equation for the reaction between the alpha particle and the aluminum atom in case (i) is written as follows-
$_{{\text{13}}}^{{\text{27}}}{\text{P + }}_{\text{2}}^{\text{4}}{\text{He}} \to _{{\text{14}}}^{{\text{30}}}{\text{Si + X}}$.
Aluminum has 13 protons and 14 neutrons (27-13=14) before the reaction with alpha particles. After the bombardment, as the number of protons as well as the neutrons in alpha particles are 2. Then the total number of protons will be 13+2=15, the total number of neutrons would be 14+2=16, the total mass number becomes 15+16=31 and the total atomic number is 15. Given that one of the products is $_{{\text{14}}}^{{\text{30}}}{\text{Si}}$, since the atomic number of Si is 14, number of protons left is 1(15-14=1) in the same manner mass number of Si is 30, only one particle is left out in the new mass number count (31-30=1) i.e. proton itself. So the species ‘x’ will be represented as $_{\text{1}}^{\text{1}}{\text{H}}$ which is named as protium, it is an isotope of hydrogen, it is a proton.
Coming to the second case where the product formed is phosphorus atom from aluminum and alpha particles, the mass number of phosphorus is 30, to add 3 particles we require two alpha particles. Let us put the chemical equation, $_{{\text{13}}}^{{\text{27}}}{\text{Al + 2}}_{\text{2}}^{\text{4}}{\text{He}} \to _{{\text{15}}}^{{\text{30}}}{\text{P + Y}}$. The new mass number is 31 (27+4=31) and atomic number is 15 (13+2=15). Given that phosphorus atomic number is 15, mass number is 30, the product ‘Y’ will have mass number 1 (31-30=1) and atomic number 0. That means the species contains only one neutron and it is a neutron itself $_{\text{0}}^{\text{1}}{\text{n}}$. The phosphorus with A=30, Z=15 formed Silicon with Z=14, A=30 and particle ‘z’. So the ‘z’ would be having Z=1 (15-14=1), A=0 (30-30=0). It consists of only one proton but no neutrons, it is called a positron (${{\text{\beta }}^{\text{ + }}}$).
So the answer for the given question is option (A) proton, neutron, positron.
Note: In the above question, ‘x, y, z’ are found from the total number of protons and neutrons formed upon addition of helium nucleus (the alpha particle). Proton consists of one positive charge, a neutron possesses one neutral charge and the positron is an antiparticle of an electron with positive charge.
Complete step by step solution:
Given that aluminum gets disintegrated in two ways by the addition of an alpha particle.
In one way $_{{\text{13}}}^{{\text{27}}}{\text{Al}}$ gives silicon ($_{{\text{14}}}^{{\text{30}}}{\text{Si}}$) and some other species marked as ‘x’ and in the second way it forms phosphorus $\left( {_{15}^{35}{\text{P}}} \right)$ and a species ‘y’ in the first case and the phosphorus in turn forms silicon-14 isotope $\left( {_{{\text{14}}}^{{\text{30}}}{\text{Si}}} \right)$.
So let us find out the unknown species ‘x’. The chemical equation for the reaction between the alpha particle and the aluminum atom in case (i) is written as follows-
$_{{\text{13}}}^{{\text{27}}}{\text{P + }}_{\text{2}}^{\text{4}}{\text{He}} \to _{{\text{14}}}^{{\text{30}}}{\text{Si + X}}$.
Aluminum has 13 protons and 14 neutrons (27-13=14) before the reaction with alpha particles. After the bombardment, as the number of protons as well as the neutrons in alpha particles are 2. Then the total number of protons will be 13+2=15, the total number of neutrons would be 14+2=16, the total mass number becomes 15+16=31 and the total atomic number is 15. Given that one of the products is $_{{\text{14}}}^{{\text{30}}}{\text{Si}}$, since the atomic number of Si is 14, number of protons left is 1(15-14=1) in the same manner mass number of Si is 30, only one particle is left out in the new mass number count (31-30=1) i.e. proton itself. So the species ‘x’ will be represented as $_{\text{1}}^{\text{1}}{\text{H}}$ which is named as protium, it is an isotope of hydrogen, it is a proton.
Coming to the second case where the product formed is phosphorus atom from aluminum and alpha particles, the mass number of phosphorus is 30, to add 3 particles we require two alpha particles. Let us put the chemical equation, $_{{\text{13}}}^{{\text{27}}}{\text{Al + 2}}_{\text{2}}^{\text{4}}{\text{He}} \to _{{\text{15}}}^{{\text{30}}}{\text{P + Y}}$. The new mass number is 31 (27+4=31) and atomic number is 15 (13+2=15). Given that phosphorus atomic number is 15, mass number is 30, the product ‘Y’ will have mass number 1 (31-30=1) and atomic number 0. That means the species contains only one neutron and it is a neutron itself $_{\text{0}}^{\text{1}}{\text{n}}$. The phosphorus with A=30, Z=15 formed Silicon with Z=14, A=30 and particle ‘z’. So the ‘z’ would be having Z=1 (15-14=1), A=0 (30-30=0). It consists of only one proton but no neutrons, it is called a positron (${{\text{\beta }}^{\text{ + }}}$).
So the answer for the given question is option (A) proton, neutron, positron.
Note: In the above question, ‘x, y, z’ are found from the total number of protons and neutrons formed upon addition of helium nucleus (the alpha particle). Proton consists of one positive charge, a neutron possesses one neutral charge and the positron is an antiparticle of an electron with positive charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




