
Bond angle present in the methane ($CH_{ 4 }$) molecule is:
A.105$^{ 0 }$
B.107$^{ 0 }$
C.109$^{ 0 }$
D.110$^{ 0 }$
Answer
592.8k+ views
Hint: Methane molecule has ${ sp }^{ 3 }$ hybridization. Now try to figure out the value for the standard bond angle in this hybridization.
Complete step by step answer:
-Let’s talk about the $CH_{ 4 }$ molecule. This is basically a combination of 1 carbon atom and 4 hydrogen atoms. However, to form this compound the central atom carbon has to complete its octet. It has 4 valence electrons and it obtains 4 more electrons from 4 hydrogen atoms. Hence, by sharing electrons between carbon and hydrogen there is a formation of a covalent bond.
-Now, we will discuss the hybridization of methane, the carbon here is ${ sp }^{ 3 }$ hybridized because one 2s orbital and three 2p orbitals in the valence shell of carbon combine to form four ${ sp }^{ 3 }$ hybrid orbitals that are of equal energy and also have equal shape. Further, four H atoms also use these four ${ sp }^{ 3 }$ hybrid orbitals of carbon to form four sigma bonds. It finally leads to the formation of the methane molecule.
-We have discussed the hybridization process. Now, determining the molecular geometry of methane should be easier for us. In methane, the four hybrid orbitals are located in such a manner so that they can decrease the force of repulsion between them. Hence, $CH_{ 4 }$ acquires a tetrahedral shape.
Hence, the ${ sp }^{ 3 }$ hybrid orbitals have a bond angle of 109$^{ 0 }$28’. We can roughly assume it to be 109$^{ 0 }$.
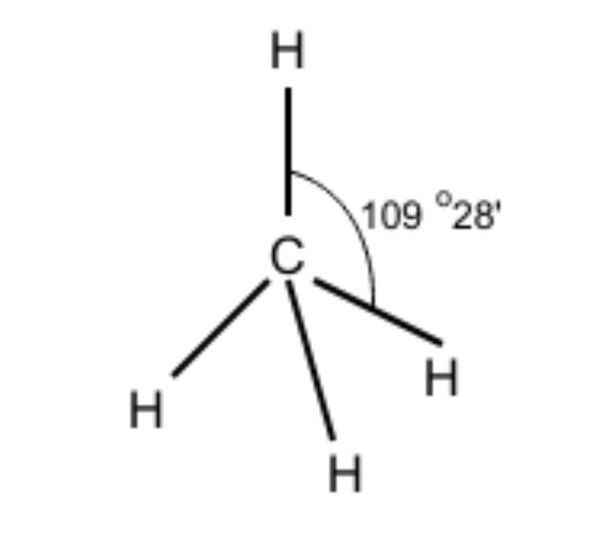
Therefore, we can conclude that the correct answer to this question is option C.
Note:
All angles are the same in this compound with a bond length between all C-H bonds equal to 1.09 Angstrom. All the outer atoms are the same - the same dipoles, and that the dipole moments are in the same direction - towards the carbon atom, the overall molecule becomes non-polar. Therefore, methane has nonpolar bonds and is nonpolar overall.
Complete step by step answer:
-Let’s talk about the $CH_{ 4 }$ molecule. This is basically a combination of 1 carbon atom and 4 hydrogen atoms. However, to form this compound the central atom carbon has to complete its octet. It has 4 valence electrons and it obtains 4 more electrons from 4 hydrogen atoms. Hence, by sharing electrons between carbon and hydrogen there is a formation of a covalent bond.
-Now, we will discuss the hybridization of methane, the carbon here is ${ sp }^{ 3 }$ hybridized because one 2s orbital and three 2p orbitals in the valence shell of carbon combine to form four ${ sp }^{ 3 }$ hybrid orbitals that are of equal energy and also have equal shape. Further, four H atoms also use these four ${ sp }^{ 3 }$ hybrid orbitals of carbon to form four sigma bonds. It finally leads to the formation of the methane molecule.
-We have discussed the hybridization process. Now, determining the molecular geometry of methane should be easier for us. In methane, the four hybrid orbitals are located in such a manner so that they can decrease the force of repulsion between them. Hence, $CH_{ 4 }$ acquires a tetrahedral shape.
Hence, the ${ sp }^{ 3 }$ hybrid orbitals have a bond angle of 109$^{ 0 }$28’. We can roughly assume it to be 109$^{ 0 }$.

Therefore, we can conclude that the correct answer to this question is option C.
Note:
All angles are the same in this compound with a bond length between all C-H bonds equal to 1.09 Angstrom. All the outer atoms are the same - the same dipoles, and that the dipole moments are in the same direction - towards the carbon atom, the overall molecule becomes non-polar. Therefore, methane has nonpolar bonds and is nonpolar overall.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




