
How many bonding orbitals are in silicon tetrafluoride or $\text{Si}{{\text{F}}_{\text{4}}}$?
Answer
549.6k+ views
Hint: In silicon tetrafluoride, there are four bonding orbital. Bounding orbital as a name indicates the attractive interaction between the atomic orbital of two or more atoms in molecules, electrons in the orbitals Stabilize a molecule.
Complete step by step answer:
Silicon tetrafluoride is a covalent molecule. It contains a central silicon atom which is bound to four fluorine atoms through four single covalent bonds. Silicon has four unpaired electrons, To fulfill its valence silicon atom gets attached to four fluorine atoms. Fluorine atoms contain one valence electron to form a covalent bond with silicon.
$4$ bonding pairs with zero non-bonding pairs on silicon atom results in a tetrahedral shape of ${{\text{S}}_{\text{1}}}{{\text{F}}_{\text{4}}}$.
Lewis Structure of $\text{Si}{{\text{F}}_{4}}$
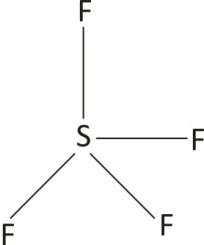
Since each single covalent bond represent a $\sigma $ orbitals there are four bonding orbitals in $\text{Si}{{\text{F}}_{4}}$
$\text{Si}{{\text{F}}_{4}}$ have $s{{p}^{3}}$ hybridization.
The bonding orbital is defined as an attractive interaction between the atomic orbital of a bonding atom in a molecule.
Note: Silicon tetrafluoride is a colorless gas with strong odor.
It is used to seal oil well from water and also used in production of silicon and fluosilicic acid.
Silicon tetrafluoride is hazardous.
Complete step by step answer:
Silicon tetrafluoride is a covalent molecule. It contains a central silicon atom which is bound to four fluorine atoms through four single covalent bonds. Silicon has four unpaired electrons, To fulfill its valence silicon atom gets attached to four fluorine atoms. Fluorine atoms contain one valence electron to form a covalent bond with silicon.
$4$ bonding pairs with zero non-bonding pairs on silicon atom results in a tetrahedral shape of ${{\text{S}}_{\text{1}}}{{\text{F}}_{\text{4}}}$.
Lewis Structure of $\text{Si}{{\text{F}}_{4}}$

Since each single covalent bond represent a $\sigma $ orbitals there are four bonding orbitals in $\text{Si}{{\text{F}}_{4}}$
$\text{Si}{{\text{F}}_{4}}$ have $s{{p}^{3}}$ hybridization.
The bonding orbital is defined as an attractive interaction between the atomic orbital of a bonding atom in a molecule.
Note: Silicon tetrafluoride is a colorless gas with strong odor.
It is used to seal oil well from water and also used in production of silicon and fluosilicic acid.
Silicon tetrafluoride is hazardous.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




