
Why is the borane-THF complex used for hydroboration?
Answer
490.8k+ views
Hint: Borane-THF complex is a charge-transfer complex. It is used in the hydroboration process because it is safe and convenient to use. The active ingredient in this complex is borane $ B{H_3} $ . The only problem with this complex is that it has the highly disagreeable smell of rotten cabbage.
Complete answer:
Borane-THF complex is a dipolar bond charge-transfer complex that consists of borane and tetrahydrofuran. Its full name is Borane-tetrahydrofuran complex. The complex can reduce carboxylic acids to alcohols and is a common route for the reduction of amino acids to amino alcohols. It is also used as a source of borane $ (B{H_3}) $ for the formation of adducts.
The Borane-THF complex is used for hydroboration because it is safe and convenient to use.
This complex is also used in the reduction of carboxylic acids to alcohols of nitriles to primary amines. It reacts with olefins to add the $ B{H_2} $ functional group.
In a solution in THF, borane exists as a loose Lewis acid-base complex. This allows the boron to have an octet and makes the reagent more stable.
The complex is commercially available but can also be generated by the dissolution of diborane in THF.
Below is the structure of the Borane-tetrahydrofuran (THF) complex.
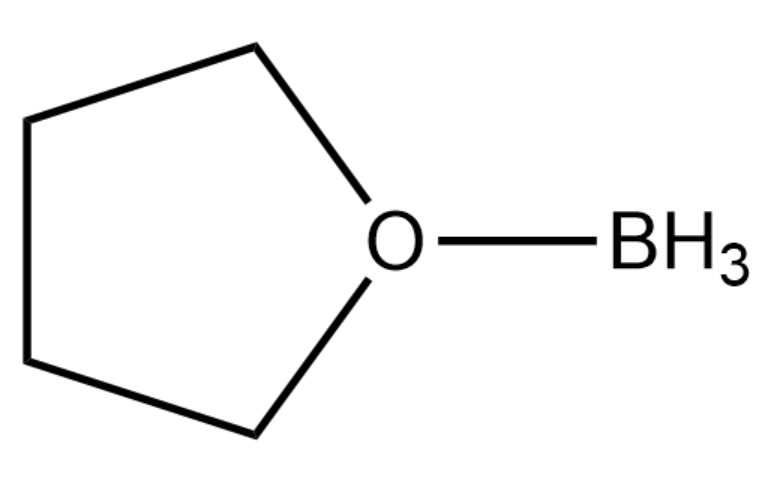
Note:
Borane exists naturally as the dimer $ {B_2}{H_6} $ (diborane), but diborane mixes easily with air and forms explosive mixtures. Also, it ignites spontaneously in moist air at room temperature. Thus, the solution is highly sensitive to air and requires the use of air-free techniques.
Complete answer:
Borane-THF complex is a dipolar bond charge-transfer complex that consists of borane and tetrahydrofuran. Its full name is Borane-tetrahydrofuran complex. The complex can reduce carboxylic acids to alcohols and is a common route for the reduction of amino acids to amino alcohols. It is also used as a source of borane $ (B{H_3}) $ for the formation of adducts.
The Borane-THF complex is used for hydroboration because it is safe and convenient to use.
This complex is also used in the reduction of carboxylic acids to alcohols of nitriles to primary amines. It reacts with olefins to add the $ B{H_2} $ functional group.
In a solution in THF, borane exists as a loose Lewis acid-base complex. This allows the boron to have an octet and makes the reagent more stable.
The complex is commercially available but can also be generated by the dissolution of diborane in THF.
Below is the structure of the Borane-tetrahydrofuran (THF) complex.

Note:
Borane exists naturally as the dimer $ {B_2}{H_6} $ (diborane), but diborane mixes easily with air and forms explosive mixtures. Also, it ignites spontaneously in moist air at room temperature. Thus, the solution is highly sensitive to air and requires the use of air-free techniques.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




