
Boric acid is a very weak acid but in the presence of certain organic compounds, it acts as a strong acid. Which one of the following organic compound(s) affect cannot such
(A) change?
(B) Glycerol
(C) Acetic acid
(D) Ethyl Alcohol
(E) Ethylene
Answer
588.9k+ views
Hint: It acts as a strong acid in presence of polyhydroxy compounds, one such compound contains three carbon atoms and one hydroxyl group is attached to each carbon atom. It is used in improving hydration. Boric acid acts as Lewis acid because it accepts lone pairs of electrons.
Complete solution step by step:
-Boric acid is a weak monobasic acid but it does not donate protons, so it is not a protonic acid.
-it acts as Lewis acid as it accepts lone pairs of electrons from hydroxyl ions. As boron has three bonds and six electrons so to complete its octet it accepts lone pairs of electrons.
-Boron itself cannot release hydrogen ion but as it receives a lone pair of electron from hydroxyl group in turn it releases hydrogen ion.

-When boric acid reacts with ethyl alcohol, it forms triethyl borate. It is an ester of boric acid and ethanol.
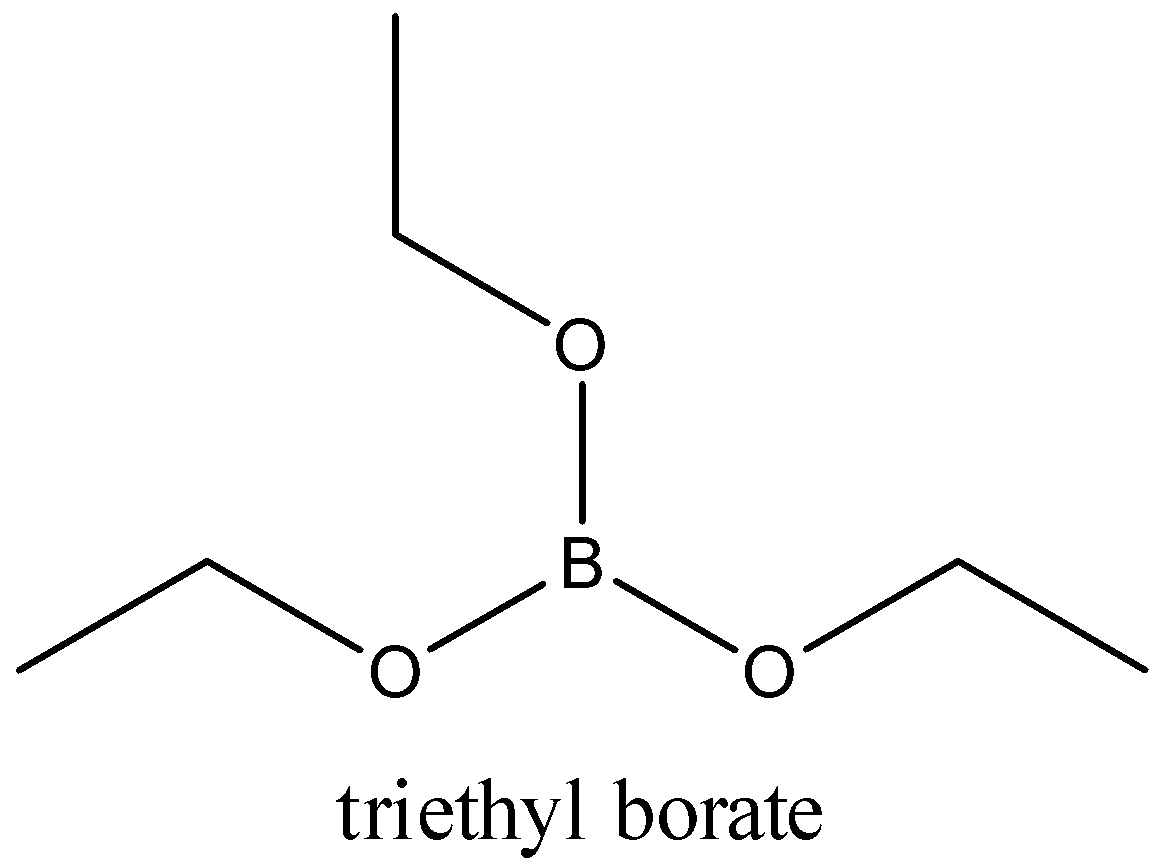
Boric acid acts as weak Lewis acid.
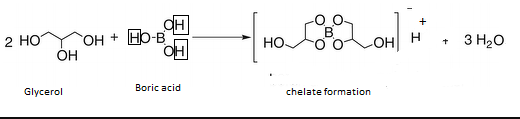
-Boric acid forms a stable cyclic complex with polyhydroxy compounds like glycerol. This helps in release of hydrogen ions.
Hence, Boric acid is a very weak acid but in the presence of certain organic compounds, it acts as a strong acid. Option (B) Acetic acid, (C)Ethyl Alcohol, (D)Ethylene Cannot affect such change.
Note: Boric acid is prepared by acidifying the solution of borax. Boric acid is sparingly soluble in water but highly soluble in hot water. Planar $B{{O}_{3}}$units are joined by hydrogen bonds and it has a layer structure. -Boron itself cannot release hydrogen ions but as it receives a lone pair of electrons from the hydroxyl group in turn it releases hydrogen ions.
Complete solution step by step:
-Boric acid is a weak monobasic acid but it does not donate protons, so it is not a protonic acid.
-it acts as Lewis acid as it accepts lone pairs of electrons from hydroxyl ions. As boron has three bonds and six electrons so to complete its octet it accepts lone pairs of electrons.
-Boron itself cannot release hydrogen ion but as it receives a lone pair of electron from hydroxyl group in turn it releases hydrogen ion.

-When boric acid reacts with ethyl alcohol, it forms triethyl borate. It is an ester of boric acid and ethanol.

Boric acid acts as weak Lewis acid.

-Boric acid forms a stable cyclic complex with polyhydroxy compounds like glycerol. This helps in release of hydrogen ions.
Hence, Boric acid is a very weak acid but in the presence of certain organic compounds, it acts as a strong acid. Option (B) Acetic acid, (C)Ethyl Alcohol, (D)Ethylene Cannot affect such change.
Note: Boric acid is prepared by acidifying the solution of borax. Boric acid is sparingly soluble in water but highly soluble in hot water. Planar $B{{O}_{3}}$units are joined by hydrogen bonds and it has a layer structure. -Boron itself cannot release hydrogen ions but as it receives a lone pair of electrons from the hydroxyl group in turn it releases hydrogen ions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




