
Boric acid is polymeric due to:
A) Its acidic nature
B) The presence of hydrogen bonds
C) Its monobasic nature
D) Its geometry
Answer
569.7k+ views
Hint:Boric acid also called hydrogen borate, boracic acid, and orthoboric acid. It is a weak, monobasic(one hydrogen as proton donor) Lewis acid of boron. Some reaction shows that it is a tribasic acid in bronsted theory.
Complete answer:
Boron can form many compounds. There are some useful compounds to borons as well. These are borax, orthoboric acid and diborane.
Borax is most important. It is white crystalline solid of formula $N{a_2}{B_4}{O_7}.10{H_2}O$ . Borax dissolves in water and gives alkaline solution. The simplest boron hydride is known as diborane, prepared by treating boron trifluoride with lithium aluminium hydride.The orthoboric acid ${H_3}B{O_3}$ is also white crystalline solid, with soapy touch. It is sparingly soluble in water but highly soluble in water. It can be prepared by acidifying an aqueous solution of borax
$N{a_2}{B_4}{O_7} + 2HCl + 5{H_2}O \to 2NaCl + 4{H_3}B{O_3}$
Structure of boric acid- Orthoboric acid has a layer structure in which planar $B{O_3}$ units are joined by hydrogen bonds. Structure of boric acid as-
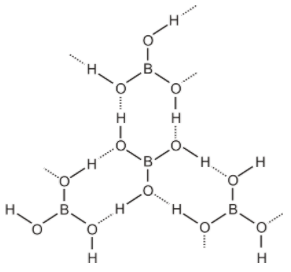
Here the dotted line represents hydrogen bonds.
Boric acid is a weak monobasic acid since it is not able to release hydrogen ion on its own, it receives hydroxyl ion from water, completes its octet and then releases hydrogen ion. It is not a protonic acid, but acts as Lewis acid by accepting an electron pair from hydroxyl ions.
On heating, orthoboric acid above $370K$ forms metaboric acid, which on further heating gives boric oxide. So the correct option is B. as we can see from the figure that it is polymeric due then hydrogen bonds.
Note:Boric acid is very useful. It can be used as antiseptic for minor burns or cuts. Also it can be used as antibacterial in acne treatment. It is effectively used to counteract the harmful effects of reactive hydrofluoric acid after contact with skin.
Complete answer:
Boron can form many compounds. There are some useful compounds to borons as well. These are borax, orthoboric acid and diborane.
Borax is most important. It is white crystalline solid of formula $N{a_2}{B_4}{O_7}.10{H_2}O$ . Borax dissolves in water and gives alkaline solution. The simplest boron hydride is known as diborane, prepared by treating boron trifluoride with lithium aluminium hydride.The orthoboric acid ${H_3}B{O_3}$ is also white crystalline solid, with soapy touch. It is sparingly soluble in water but highly soluble in water. It can be prepared by acidifying an aqueous solution of borax
$N{a_2}{B_4}{O_7} + 2HCl + 5{H_2}O \to 2NaCl + 4{H_3}B{O_3}$
Structure of boric acid- Orthoboric acid has a layer structure in which planar $B{O_3}$ units are joined by hydrogen bonds. Structure of boric acid as-

Here the dotted line represents hydrogen bonds.
Boric acid is a weak monobasic acid since it is not able to release hydrogen ion on its own, it receives hydroxyl ion from water, completes its octet and then releases hydrogen ion. It is not a protonic acid, but acts as Lewis acid by accepting an electron pair from hydroxyl ions.
On heating, orthoboric acid above $370K$ forms metaboric acid, which on further heating gives boric oxide. So the correct option is B. as we can see from the figure that it is polymeric due then hydrogen bonds.
Note:Boric acid is very useful. It can be used as antiseptic for minor burns or cuts. Also it can be used as antibacterial in acne treatment. It is effectively used to counteract the harmful effects of reactive hydrofluoric acid after contact with skin.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




