
Boyle’s temperature or Boyle point is the temperature at which a real gas starts behaving like an ideal gas over a particular range of pressure. A graph is plotted between the compressibility factor Z and pressure P.
What is the variation of Z with P?
(A) At very low pressures, all gases show Z$\approx 1$
(B) At high pressures, all gases show Z>1
(C) At intermediate pressures, all gases show Z<1
(D) All of the above
Answer
587.7k+ views
Hint: Compressibility factor is a very important factor in real gases as this is the factor that determines the deviation of real gases from ideal behaviour. It is denoted by Z. It is shown in two forms – variation with pressure at constant temperature and variation with pressure at different temperatures.
Complete step by step solution:
-Real gases do not obey the ideal gas laws due to the assumptions of the ideal gases which are
1. Real gas molecules have a finite volume.
2. We cannot neglect the intermolecular attractive forces between real gas molecules which is considered zero for ideal gases.
-The formula derived for ideal gases is ${{\left( PV \right)}_{ideal}}=nRT$ which is called the gas law. Real gases do not follow this law. The deviation of real gases is studied by compressibility factor (Z) which can be represented as
$\begin{align}
& Z=\dfrac{{{\left( PV \right)}_{real}}}{{{\left( PV \right)}_{ideal}}} \\
& Z=\dfrac{PV}{nRT}=\dfrac{P{{V}_{m}}}{RT} \\
\end{align}$
Where ${{V}_{m}}$ is the molar volume or volume of 1 mole of gas.
-Variation of Z with pressure can be shown by a graph as
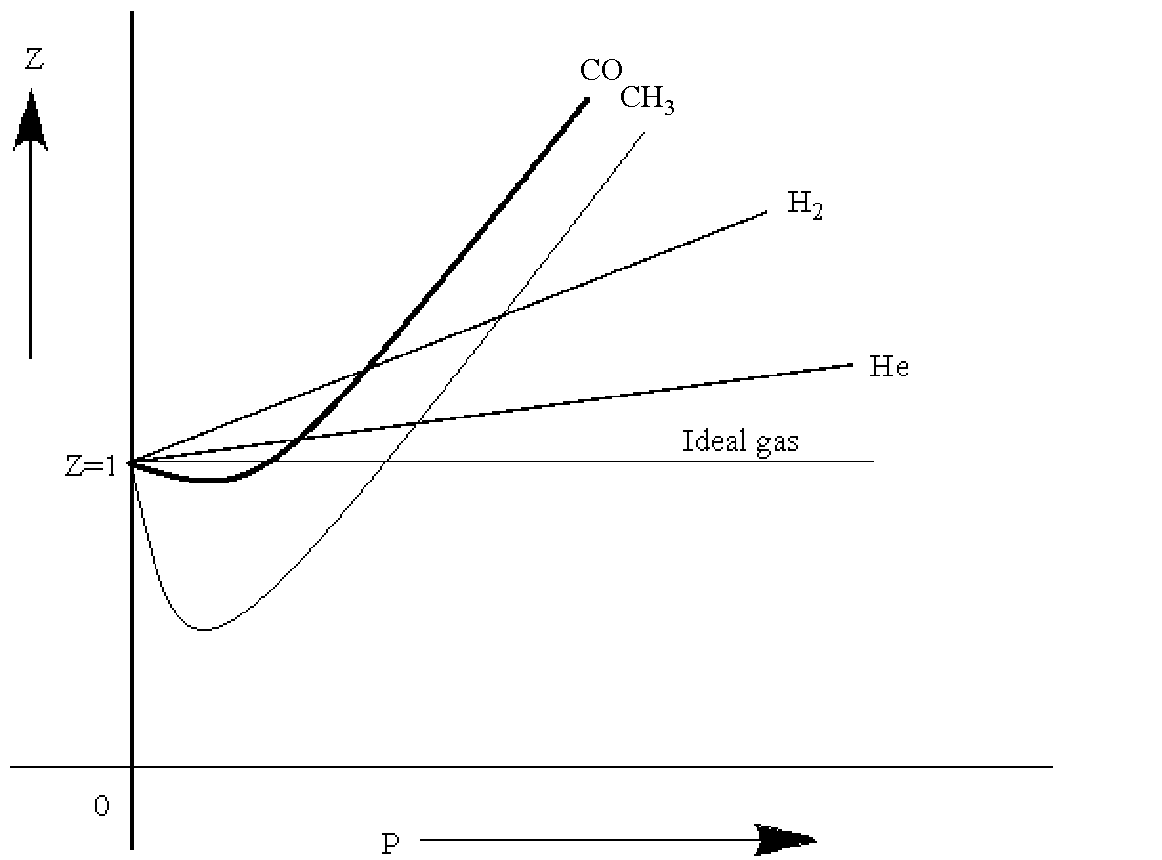
-Thus by seeing this graph, we can conclude,
Z=1 for ideal gas.
Z>1 at all pressures for He/${{H}_{2}}$
Z<1 at low pressures.(for all other gases)
Z>1 at high pressures (for all other gases)
Therefore, the correct option is B. At high pressures, all gases show Z>1.
Note: Only carbon-monoxide and methane have Z<1 for certain pressure ranges. All other gases have Z>1 for all values of pressure. As the temperature of a gas is increased, it comes nearer to the ideal gas behavior as the intermolecular forces of attraction become weaker and weaker with the increase in heat.
Complete step by step solution:
-Real gases do not obey the ideal gas laws due to the assumptions of the ideal gases which are
1. Real gas molecules have a finite volume.
2. We cannot neglect the intermolecular attractive forces between real gas molecules which is considered zero for ideal gases.
-The formula derived for ideal gases is ${{\left( PV \right)}_{ideal}}=nRT$ which is called the gas law. Real gases do not follow this law. The deviation of real gases is studied by compressibility factor (Z) which can be represented as
$\begin{align}
& Z=\dfrac{{{\left( PV \right)}_{real}}}{{{\left( PV \right)}_{ideal}}} \\
& Z=\dfrac{PV}{nRT}=\dfrac{P{{V}_{m}}}{RT} \\
\end{align}$
Where ${{V}_{m}}$ is the molar volume or volume of 1 mole of gas.
-Variation of Z with pressure can be shown by a graph as

-Thus by seeing this graph, we can conclude,
Z=1 for ideal gas.
Z>1 at all pressures for He/${{H}_{2}}$
Z<1 at low pressures.(for all other gases)
Z>1 at high pressures (for all other gases)
Therefore, the correct option is B. At high pressures, all gases show Z>1.
Note: Only carbon-monoxide and methane have Z<1 for certain pressure ranges. All other gases have Z>1 for all values of pressure. As the temperature of a gas is increased, it comes nearer to the ideal gas behavior as the intermolecular forces of attraction become weaker and weaker with the increase in heat.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




