
Bredig’s arc method is used for the preparation of colloidal solution of:
A. Metals like, silver, gold, etc.
B. Organic compounds
C. Two liquids
D. Inorganic compounds
Answer
609.6k+ views
Hint: We use this method for the preparation of noble metals which are stable in nature. They are “precious” metals, meaning their occurrence in the earth’s crust is rare.
Complete step by step answer:
The method of preparation of colloidal solutions of metals such as gold or silver is known as Bredig's arc method.
From this above definition we find out that option is A might be our correct answer. Now, for confirmation let’s talk about more Bredig’s arc method.
This method consists of both dispersion and condensation. In this method colloidal sols of metals such as gold, silver can be prepared.
For example, if we want to make a colloidal solution silver then it is prepared by condensation of vapours. We can do this by striking an electric arc between electrodes, under the surface of water containing some stabilizing agent such as traces of potassium hydroxide.
An arc is struck between electrodes, under the surface of water containing some stabilizing agent (traces of potassium hydroxide). The intense heat of the arc vaporizes some of the metal which then condenses under cold water. The water is kept cold as an ice bath. The colloidal particle prepared is stabilised by adding a small amount of potassium hydroxide to it. The intense heat produced from that arc tears off the end of silver rods to form vapour of silver which then condenses to give particles of colloidal size. Presence of KOH stabilizes the sol so formed.
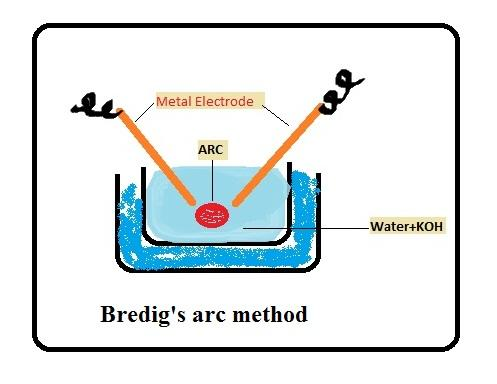
Now after studying Bredig's arc method, we are now confirmed that our correct option is A. because from the above description, it is now clear that Bredig’s arc method is used for the preparation of colloids of metals like gold and silver. So, our correct answer is option A.
Note: We should know that colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. Study of gold nanoparticles is the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine. It will be very interesting to study about its different properties.
Complete step by step answer:
The method of preparation of colloidal solutions of metals such as gold or silver is known as Bredig's arc method.
From this above definition we find out that option is A might be our correct answer. Now, for confirmation let’s talk about more Bredig’s arc method.
This method consists of both dispersion and condensation. In this method colloidal sols of metals such as gold, silver can be prepared.
For example, if we want to make a colloidal solution silver then it is prepared by condensation of vapours. We can do this by striking an electric arc between electrodes, under the surface of water containing some stabilizing agent such as traces of potassium hydroxide.
An arc is struck between electrodes, under the surface of water containing some stabilizing agent (traces of potassium hydroxide). The intense heat of the arc vaporizes some of the metal which then condenses under cold water. The water is kept cold as an ice bath. The colloidal particle prepared is stabilised by adding a small amount of potassium hydroxide to it. The intense heat produced from that arc tears off the end of silver rods to form vapour of silver which then condenses to give particles of colloidal size. Presence of KOH stabilizes the sol so formed.

Now after studying Bredig's arc method, we are now confirmed that our correct option is A. because from the above description, it is now clear that Bredig’s arc method is used for the preparation of colloids of metals like gold and silver. So, our correct answer is option A.
Note: We should know that colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. Study of gold nanoparticles is the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine. It will be very interesting to study about its different properties.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




