
How would you bring the Acetic acid methylamine conversion?
Answer
497.4k+ views
Hint: In the conversion reaction, a reactant gets converted into a desired product. These types of reactions take place in the presence of some reagent. A reagent is a chemical compound which helps a reaction to proceed in a desired direction to form a desired product. So, we will see the step by step procedure of converting acetic acid into methylamine using different reagents.
Complete answer:
Acetic acid is a carboxylic acid with two carbon atoms and methylamine is an amine with only one carbon atom. So, we need to degrade the carbon chain from two carbon atoms to one carbon atom.
In order to convert acetic acid into methylamine, we need to proceed in a step by step manner. Now, we will look at the steps by which acetic acid is converted to methylamine.
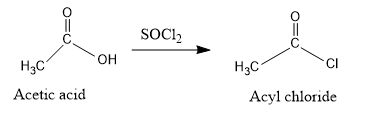
- Acetic acid is first reacted with $SOC{l_2}$ to form acyl chloride which has a good leaving group (chloride ion).
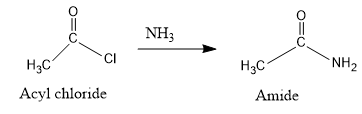
- Acyl chloride is then treated with ammonia to form an amide.
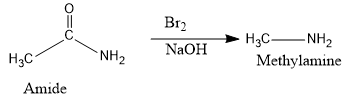
- Now, the Hoffmann degradation reaction converts the above amide into amine (methylamine).
Therefore, in this way, acetic acid is converted into methylamine.
Note:
We should remember that in Hoffmann bromamide degradation reaction, an amide is reacted with bromine and ethanolic or aqueous solution of sodium hydroxide (NaOH) which leads to the degradation of amide and formation of a primary amine. In this reaction the amine formed has one less carbon atom than the amide from which it is formed.
Complete answer:
Acetic acid is a carboxylic acid with two carbon atoms and methylamine is an amine with only one carbon atom. So, we need to degrade the carbon chain from two carbon atoms to one carbon atom.
In order to convert acetic acid into methylamine, we need to proceed in a step by step manner. Now, we will look at the steps by which acetic acid is converted to methylamine.
- Acetic acid is first reacted with $SOC{l_2}$ to form acyl chloride which has a good leaving group (chloride ion).

- Acyl chloride is then treated with ammonia to form an amide.

- Now, the Hoffmann degradation reaction converts the above amide into amine (methylamine).

Therefore, in this way, acetic acid is converted into methylamine.
Note:
We should remember that in Hoffmann bromamide degradation reaction, an amide is reacted with bromine and ethanolic or aqueous solution of sodium hydroxide (NaOH) which leads to the degradation of amide and formation of a primary amine. In this reaction the amine formed has one less carbon atom than the amide from which it is formed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




