
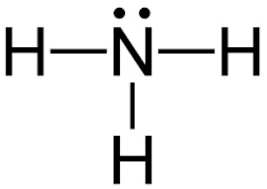
By looking at the Lewis dot structure of ammonia (\[N{H_3}\]) molecule, the shape of the ammonia molecule is described by which one of the following?
(a).Trigonal pyramidal
(b).T-shaped
(c).Square planar
(d).Trigonal planar
(e).Tetrahedral

Answer
520.2k+ views
Hint: \[N{H_3}\] is an \[s{p^3}\] hybridised molecule. Three hydrogens are bonded to the central atom nitrogen by sigma bonds and the central atom ammonia also has a lone pair of electrons.
Complete step by step answer:
The central atom in Ammonia, i.e. Nitrogen is $s{p^3}$ hybridized. It is a gas at normal conditions and is known as Azane. The molecular weight of Ammonia is 17 g/mol. It is a colourless alkaline gas.
Starting with the Lewis dot structure of Ammonia, Nitrogen has 5 valence electrons and each hydrogen has 1 valence electron. So, the total valence electrons are 8. Hydrogen always goes on the outside, so Nitrogen is the central atom. After the three valence electrons of Nitrogen have bonded with three Hydrogens, we still have two valence electrons left, which make up one lone pair of Nitrogen.

To understand the hybridization of Nitrogen in ammonia, we need to look into the configuration of nitrogen. Nitrogen in its ground state has configuration $1{s^2}2{s^2}2p{}^3$

During hybridization, one s orbital and 3 p orbitals of nitrogen hybridize to form four hybrid orbitals having equal energy levels, thus making its hybridization $s{p^3}$. Half three filled $s{p^3}$ orbitals of nitrogen form a bond with three hydrogens. The fourth fully filled hybridized orbital holds the lone pair of nitrogen.
Ammonia has a trigonal pyramidal or distorted tetrahedral structure due to the repulsive lone pair – bond pair interaction. Also, the bond angle in ammonia is less than standard $109^\circ 73'$ due to the same reason. The bond angle is $107^\circ$.
Hence, the correct answer is (A) Trigonal pyramidal.
Note: A student might not consider the lone pair of nitrogen in the hybridization of the central atom of Ammonia. The hybridisation in that case would come out to be $s{p^2}$.
Complete step by step answer:
The central atom in Ammonia, i.e. Nitrogen is $s{p^3}$ hybridized. It is a gas at normal conditions and is known as Azane. The molecular weight of Ammonia is 17 g/mol. It is a colourless alkaline gas.
Starting with the Lewis dot structure of Ammonia, Nitrogen has 5 valence electrons and each hydrogen has 1 valence electron. So, the total valence electrons are 8. Hydrogen always goes on the outside, so Nitrogen is the central atom. After the three valence electrons of Nitrogen have bonded with three Hydrogens, we still have two valence electrons left, which make up one lone pair of Nitrogen.

To understand the hybridization of Nitrogen in ammonia, we need to look into the configuration of nitrogen. Nitrogen in its ground state has configuration $1{s^2}2{s^2}2p{}^3$

During hybridization, one s orbital and 3 p orbitals of nitrogen hybridize to form four hybrid orbitals having equal energy levels, thus making its hybridization $s{p^3}$. Half three filled $s{p^3}$ orbitals of nitrogen form a bond with three hydrogens. The fourth fully filled hybridized orbital holds the lone pair of nitrogen.
Ammonia has a trigonal pyramidal or distorted tetrahedral structure due to the repulsive lone pair – bond pair interaction. Also, the bond angle in ammonia is less than standard $109^\circ 73'$ due to the same reason. The bond angle is $107^\circ$.
Hence, the correct answer is (A) Trigonal pyramidal.
Note: A student might not consider the lone pair of nitrogen in the hybridization of the central atom of Ammonia. The hybridisation in that case would come out to be $s{p^2}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




