
How could you calculate formal charge of $C{{O}_{2}}$ ?
Answer
564.9k+ views
Hint As we know that formal charge is the charge which is basically assigned to an atom in a molecule, by assuming that electrons are equally shared in all chemical bonds between atoms, irrespective of the relative electronegativity.
complete Step by step solution:
- As we know that formal charge is mainly used to predict the structure of the molecule.
- We can draw the three resonating structures possible for $C{{O}_{2}}$ as :
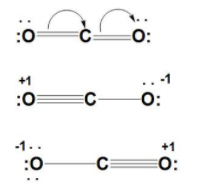
- As carbon dioxide is a neutral molecule and has 16 valence electrons. We can see the three ways possible.
- First, carbon which is bonded to one oxygen atom and double bonded to another. That is carbon has +1 charge, oxygen double=0, and oxygen single have charge -1. Hence, total formal charge is 0.
- Second, carbon that is single bonded to both the oxygen atoms. That is, carbon has +2 charge, oxygen has charge -1 each. Hence, total formal charge is 0.
- Third, carbon that is bonded to both oxygen atoms. That is carbon, oxygen both have no charges. Hence, total formal charge is 0.
- Hence, we can say that the formal charge of $C{{O}_{2}}$ is zero.
Note:
- As we know that carbon dioxide becomes a poisonous gas when it is inhaled in large quantities. It is found that this can cause severe damage to the nervous system and also can lead to many of the disorders like respiratory disorders.
- It is also found that $C{{O}_{2}}$ in the form of liquid, carbon dioxide is used for cooling and refrigeration.
complete Step by step solution:
- As we know that formal charge is mainly used to predict the structure of the molecule.
- We can draw the three resonating structures possible for $C{{O}_{2}}$ as :

- As carbon dioxide is a neutral molecule and has 16 valence electrons. We can see the three ways possible.
- First, carbon which is bonded to one oxygen atom and double bonded to another. That is carbon has +1 charge, oxygen double=0, and oxygen single have charge -1. Hence, total formal charge is 0.
- Second, carbon that is single bonded to both the oxygen atoms. That is, carbon has +2 charge, oxygen has charge -1 each. Hence, total formal charge is 0.
- Third, carbon that is bonded to both oxygen atoms. That is carbon, oxygen both have no charges. Hence, total formal charge is 0.
- Hence, we can say that the formal charge of $C{{O}_{2}}$ is zero.
Note:
- As we know that carbon dioxide becomes a poisonous gas when it is inhaled in large quantities. It is found that this can cause severe damage to the nervous system and also can lead to many of the disorders like respiratory disorders.
- It is also found that $C{{O}_{2}}$ in the form of liquid, carbon dioxide is used for cooling and refrigeration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




