
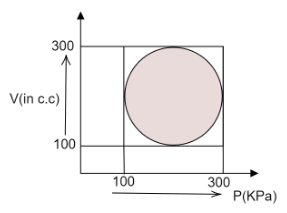
Calculate heat absorbed by a system is going through the cyclic process as shown in the figure.

Answer
563.1k+ views
Hint: In a cyclic process, the internal energy in a cycle remains constant. This makes the change in the internal energy of the system zero. This means that the heat absorbed by the system is fully used to do the work. Thus, the area of this curve gives us the heat absorbed by the system.
Complete Step by step solution:
In the question, it is given that,
The process is cyclic, therefore the change in internal energy is zero. This makes the total heat absorbed by the system equal to the area of the curve formed by the P-V curve.
From the figure, we can deduce that,
The minimum volume of the system, $ {V_i} = 100cc $
The maximum volume of the system, $ {V_f} = 300cc $
The minimum pressure of the system, $ {P_i} = 100KPa $
The maximum pressure of the system, $ {P_f} = 300KPa $
This gives, the difference between the maximum and minimum pressure,
$ \Delta P = {P_f} - {P_i} $
$ \Delta P = 300 - 100 $
$ \Delta P = 200KPa $
And the difference between the maximum and minimum volume,
$ \Delta V = {V_f} - {V_i} $
$ \Delta V = 300 - 100 $
$ \Delta V = 200cc $ or $ c{m^3} $
The range of pressure and volume change is equal,
Therefore, the curve traced by the cyclic process is circular.
We know that the area of a circle is,
$ A = \pi {r^2} $
The difference between the minimum and maximum value gives the diameter of the circle formed by the curve.
Therefore the radius of the circle,
$ r = \dfrac{d}{2} $
The area enclosed by the graph is given by,
$ A = \pi \left( {\dfrac{{\Delta V}}{2}} \right)c{m^3} \times \left( {\dfrac{{\Delta P}}{2}} \right)KPa $
Keeping the values,
$ A = \pi \left( {\dfrac{{200}}{2}} \right)c{m^3} \times \left( {\dfrac{{200}}{2}} \right)KPa $
$ \Rightarrow A = \pi \times 100c{m^3} \times 100KPa $
$ \Rightarrow A = 3.14 \times {10^4}\left( {c{m^3} \times KPa} \right) $
The units $ c{m^3} $ and $ \;KPa $ are not the SI unit values of volume and Pressure, thus, to get the answer in joules, we convert them into SI units.
We know that,
$ 1c{m^3} = {10^{ - 6}}{m^3} $
And, $ 1KPa = {10^3}Pa $
Substituting these values in the area of the curve, we get-
$ A = 3.14 \times {10^4} \times {10^{ - 6}} \times {10^3}\left( {{m^3} \times Pa} \right) $
$ A = 3.14 \times 10{m^3}Pa $
Or
$ A = 31.4J $
This is equal to the heat absorbed during the process,
$ \Delta H = 31.4J $
The heat absorbed during this cyclic process is $ 31.4\;J $
Note:
Change in internal energy is independent of the path taken by the process. Which means that no matter what path is followed, change in internal energy will only be the difference of initial and final values. Whereas, the energy absorbed or given by the system is path-dependent. This is why, at the end of a cyclic process, there is a change in the heat but no change in the internal energy.
Complete Step by step solution:
In the question, it is given that,
The process is cyclic, therefore the change in internal energy is zero. This makes the total heat absorbed by the system equal to the area of the curve formed by the P-V curve.
From the figure, we can deduce that,
The minimum volume of the system, $ {V_i} = 100cc $
The maximum volume of the system, $ {V_f} = 300cc $
The minimum pressure of the system, $ {P_i} = 100KPa $
The maximum pressure of the system, $ {P_f} = 300KPa $
This gives, the difference between the maximum and minimum pressure,
$ \Delta P = {P_f} - {P_i} $
$ \Delta P = 300 - 100 $
$ \Delta P = 200KPa $
And the difference between the maximum and minimum volume,
$ \Delta V = {V_f} - {V_i} $
$ \Delta V = 300 - 100 $
$ \Delta V = 200cc $ or $ c{m^3} $
The range of pressure and volume change is equal,
Therefore, the curve traced by the cyclic process is circular.
We know that the area of a circle is,
$ A = \pi {r^2} $
The difference between the minimum and maximum value gives the diameter of the circle formed by the curve.
Therefore the radius of the circle,
$ r = \dfrac{d}{2} $
The area enclosed by the graph is given by,
$ A = \pi \left( {\dfrac{{\Delta V}}{2}} \right)c{m^3} \times \left( {\dfrac{{\Delta P}}{2}} \right)KPa $
Keeping the values,
$ A = \pi \left( {\dfrac{{200}}{2}} \right)c{m^3} \times \left( {\dfrac{{200}}{2}} \right)KPa $
$ \Rightarrow A = \pi \times 100c{m^3} \times 100KPa $
$ \Rightarrow A = 3.14 \times {10^4}\left( {c{m^3} \times KPa} \right) $
The units $ c{m^3} $ and $ \;KPa $ are not the SI unit values of volume and Pressure, thus, to get the answer in joules, we convert them into SI units.
We know that,
$ 1c{m^3} = {10^{ - 6}}{m^3} $
And, $ 1KPa = {10^3}Pa $
Substituting these values in the area of the curve, we get-
$ A = 3.14 \times {10^4} \times {10^{ - 6}} \times {10^3}\left( {{m^3} \times Pa} \right) $
$ A = 3.14 \times 10{m^3}Pa $
Or
$ A = 31.4J $
This is equal to the heat absorbed during the process,
$ \Delta H = 31.4J $
The heat absorbed during this cyclic process is $ 31.4\;J $
Note:
Change in internal energy is independent of the path taken by the process. Which means that no matter what path is followed, change in internal energy will only be the difference of initial and final values. Whereas, the energy absorbed or given by the system is path-dependent. This is why, at the end of a cyclic process, there is a change in the heat but no change in the internal energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




