
How much \[Ca{\left( {N{O_3}} \right)_2}\], in \[mg\], must be in \[50\,ml\] of a solution with \[2.35\,ppm\] of \[Ca\]?
A. 0.1175
B. 770.8
C. 4.7
D. 0.48
Answer
583.2k+ views
Hint: A solution is marked by the presence of two parts. One part is the solute, and the other part is called the solvent. The solute is the substance that is dissolved in the solution. The solvent is the part where the solute is allowed to dissolve.
Complete step by step answer:
Given,
Volume of the solution is =\[50\,ml\]
Amount of \[Ca\] present = \[2.35\,ppm\]
Now we will have to calculate the amount of \[Ca{\left( {N{O_3}} \right)_2}\], in \[mg\] present.
We know that, for \[2.35\,ppm\] of \[Ca\],
\[\;{10^{ - 6}}{\text{ml}} = {\text{ }}2.35{\text{ mg}}\,{\text{of}}\,{\text{Ca}}\]
So, for \[50\,ml\],
\[
2.35\,{\text{ppm = }}\dfrac{{{\text{mass}}\,{\text{of}}\,{\text{solute}}\,\,{\text{in}}\,\,{\text{mg}}}}{{{\text{volume}}\,{\text{of}}\,{\text{solution}}\,\,{\text{in}}\,\,{\text{litres}}}} \\
\therefore 2.35\,{\text{ppm}} = \,\dfrac{{{\text{mass}}\,{\text{of}}\,{\text{solute}}\,\,{\text{in}}\,\,{\text{mg}}}}{{50 \times {{10}^{ - 6}}}} \\
\Rightarrow {\text{mass}}\,{\text{of}}\,{\text{solute}}\,\,{\text{in}}\,\,{\text{mg = }}\,2.35 \times 50 \times {10^{ - 6}} \\
= \,117.5 \times {10^{ - 6}} \\
\]
Now, we will have to calculate the molar mass of \[Ca{\left( {N{O_3}} \right)_2}\]
Molar mass of \[Ca{\left( {N{O_3}} \right)_2}\]=\[40 + 28 + 96 = 164\,{\text{grams}}\]
Now, we will calculate the mass of \[Ca{\left( {N{O_3}} \right)_2}\]
\[
164\,{\text{g}}\,{\text{of}}\,{\text{Ca}}{\left( {{\text{N}}{{\text{O}}_{\text{3}}}} \right)_{\text{2}}} = 40\,{\text{g}}\,{\text{of}}\,{\text{Ca}} \\
= \,117.5 \times {10^{ - 6}} \times 164\,{\text{g}}\,{\text{of}}\,{\text{Ca}}{\left( {{\text{N}}{{\text{O}}_{\text{3}}}} \right)_{\text{2}}} \\
= \,481.75\,{\text{g}} \\
= 481.75 \times {10^{ - 3}}\,{\text{mg}} \\
= \,0.48\, \\
\]
Therefore, out of the given four options, (D) is the correct option. A, B and C are incorrect options.
Additional information:
Calcium nitrate is colourless in nature. This compound absorbs or takes up moisture from the surrounding air and so it is found in the form of tetrahydrate. It is one of the most important components present in the fertilizers.
We can produce calcium nitrate by various chemical methods. Some methods of the preparation of calcium nitrate are given below.
\[{\text{Ca}}{\left( {{\text{N}}{{\text{O}}_{\text{3}}}} \right)_{\text{2}}} + {\text{2HN}}{{\text{O}}_{\text{3}}} \to {\text{Ca}}{\left( {{\text{N}}{{\text{O}}_{\text{3}}}} \right)_{\text{2}}} + {\text{C}}{{\text{O}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
Here, calcium nitrate is prepared from the reaction of lime-stone with that of nitric acid, It is followed by neutralization.
Another method of its preparation is by the Odda process. The reaction of this preparation is given as follows.
\[{\text{C}}{{\text{a}}_{\text{3}}}{\left( {{\text{P}}{{\text{O}}_{\text{4}}}} \right)_{\text{2}}} + 6{\text{HN}}{{\text{O}}_{\text{3}}} + 1{\text{2}}{{\text{H}}_{\text{2}}}{\text{O}} \to 2{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}} + 3{\text{Ca}}{\left( {{\text{N}}{{\text{O}}_{\text{3}}}} \right)_{\text{2}}} + 12{{\text{H}}_{\text{2}}}{\text{O}}\]
Note:Calcium nitrate has the chemical formula \[{\text{Ca}}{\left( {{\text{N}}{{\text{O}}_{\text{3}}}} \right)_{\text{2}}}\]. This compound is also called Norges Saltpeter. The structure of this compound is written as follows.
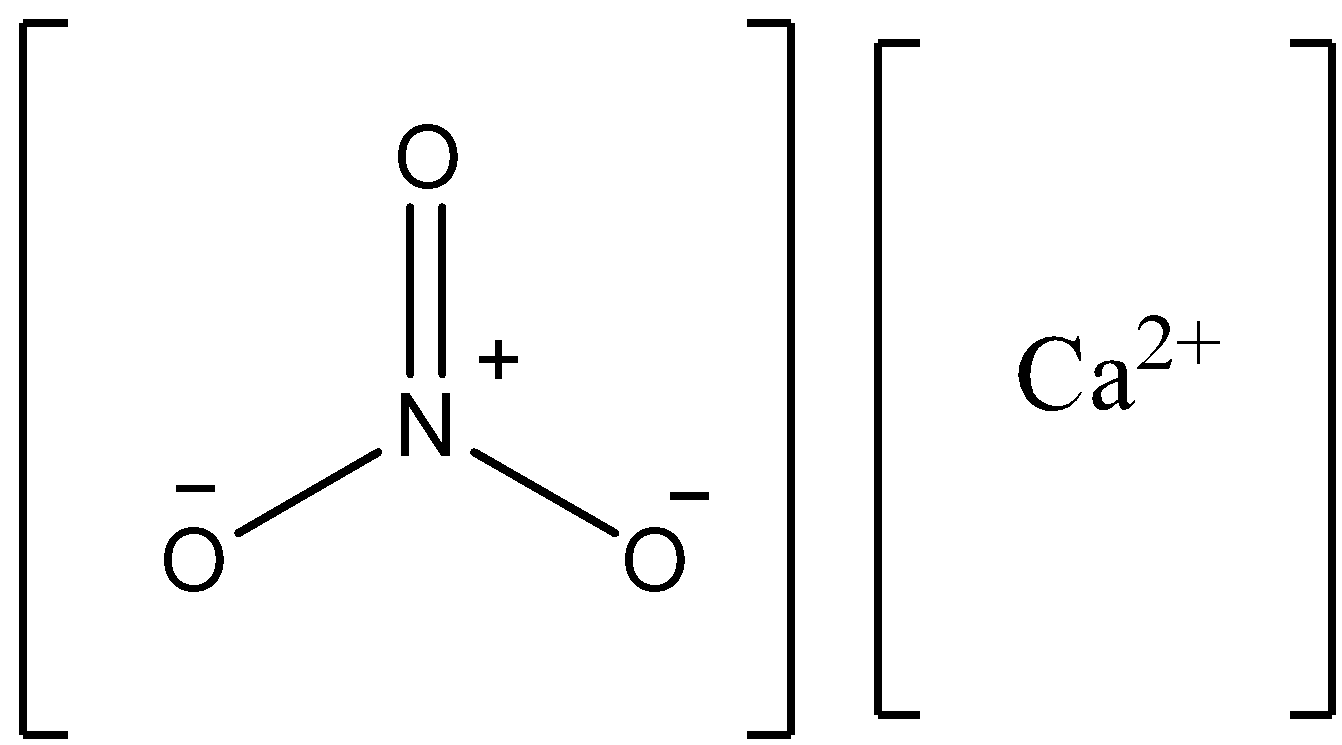
Complete step by step answer:
Given,
Volume of the solution is =\[50\,ml\]
Amount of \[Ca\] present = \[2.35\,ppm\]
Now we will have to calculate the amount of \[Ca{\left( {N{O_3}} \right)_2}\], in \[mg\] present.
We know that, for \[2.35\,ppm\] of \[Ca\],
\[\;{10^{ - 6}}{\text{ml}} = {\text{ }}2.35{\text{ mg}}\,{\text{of}}\,{\text{Ca}}\]
So, for \[50\,ml\],
\[
2.35\,{\text{ppm = }}\dfrac{{{\text{mass}}\,{\text{of}}\,{\text{solute}}\,\,{\text{in}}\,\,{\text{mg}}}}{{{\text{volume}}\,{\text{of}}\,{\text{solution}}\,\,{\text{in}}\,\,{\text{litres}}}} \\
\therefore 2.35\,{\text{ppm}} = \,\dfrac{{{\text{mass}}\,{\text{of}}\,{\text{solute}}\,\,{\text{in}}\,\,{\text{mg}}}}{{50 \times {{10}^{ - 6}}}} \\
\Rightarrow {\text{mass}}\,{\text{of}}\,{\text{solute}}\,\,{\text{in}}\,\,{\text{mg = }}\,2.35 \times 50 \times {10^{ - 6}} \\
= \,117.5 \times {10^{ - 6}} \\
\]
Now, we will have to calculate the molar mass of \[Ca{\left( {N{O_3}} \right)_2}\]
Molar mass of \[Ca{\left( {N{O_3}} \right)_2}\]=\[40 + 28 + 96 = 164\,{\text{grams}}\]
Now, we will calculate the mass of \[Ca{\left( {N{O_3}} \right)_2}\]
\[
164\,{\text{g}}\,{\text{of}}\,{\text{Ca}}{\left( {{\text{N}}{{\text{O}}_{\text{3}}}} \right)_{\text{2}}} = 40\,{\text{g}}\,{\text{of}}\,{\text{Ca}} \\
= \,117.5 \times {10^{ - 6}} \times 164\,{\text{g}}\,{\text{of}}\,{\text{Ca}}{\left( {{\text{N}}{{\text{O}}_{\text{3}}}} \right)_{\text{2}}} \\
= \,481.75\,{\text{g}} \\
= 481.75 \times {10^{ - 3}}\,{\text{mg}} \\
= \,0.48\, \\
\]
Therefore, out of the given four options, (D) is the correct option. A, B and C are incorrect options.
Additional information:
Calcium nitrate is colourless in nature. This compound absorbs or takes up moisture from the surrounding air and so it is found in the form of tetrahydrate. It is one of the most important components present in the fertilizers.
We can produce calcium nitrate by various chemical methods. Some methods of the preparation of calcium nitrate are given below.
\[{\text{Ca}}{\left( {{\text{N}}{{\text{O}}_{\text{3}}}} \right)_{\text{2}}} + {\text{2HN}}{{\text{O}}_{\text{3}}} \to {\text{Ca}}{\left( {{\text{N}}{{\text{O}}_{\text{3}}}} \right)_{\text{2}}} + {\text{C}}{{\text{O}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
Here, calcium nitrate is prepared from the reaction of lime-stone with that of nitric acid, It is followed by neutralization.
Another method of its preparation is by the Odda process. The reaction of this preparation is given as follows.
\[{\text{C}}{{\text{a}}_{\text{3}}}{\left( {{\text{P}}{{\text{O}}_{\text{4}}}} \right)_{\text{2}}} + 6{\text{HN}}{{\text{O}}_{\text{3}}} + 1{\text{2}}{{\text{H}}_{\text{2}}}{\text{O}} \to 2{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}} + 3{\text{Ca}}{\left( {{\text{N}}{{\text{O}}_{\text{3}}}} \right)_{\text{2}}} + 12{{\text{H}}_{\text{2}}}{\text{O}}\]
Note:Calcium nitrate has the chemical formula \[{\text{Ca}}{\left( {{\text{N}}{{\text{O}}_{\text{3}}}} \right)_{\text{2}}}\]. This compound is also called Norges Saltpeter. The structure of this compound is written as follows.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




