
Canonical structure of anilinium ion obtained by accepting a proton are given below. Choose the correct statements.
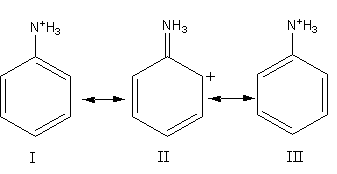
A. Anilinium ion has stable canonical structures I and III.
B. II is not an acceptable structure because carbonium ion is less stable.
C. Only I and III are acceptable aromatic canonical structures since II is non-aromatic.
D. Anilinium ion has three stable canonical structures I, II and III.

Answer
558.9k+ views
Hint: To answer this question we should know the concept of resonance. In resonating structure only the arrangement of electrons differ, bonding and positions of atoms remain the same. All possible resonating structures follow the octet rule.
Complete step-by-step answer:
Resonance is the concept according to which a structure can be shown by more than one Lewis structure. All these structures are known as resonance structures or canonical structures.
The characteristics of resonance are as follows:
-The uncharged structure is more stable than the charged.
-Charge separation remains low. More electronegative atom bears negative charge and a more electropositive atom bears a positive charge.
-Equivalent resonating structures mean the delocalization of change at the same atoms, which are more stable resonating structures.
In structures I and III electrons are delocalized among all carbon atoms. Position of bonds and atoms are the same and the electrons are delocalized in form of double bond. Octet of each atom is complete. So, I and II are stable resonating structures.
In structure II, the electronic configuration of nitrogen is ${\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{3}}}$ . The valance electrons of nitrogen is $5$. It gets three electrons from three hydrogen atoms and two electrons from double bonds, so the total valence electrons of the nitrogen is $10$. Nitrogen does not have d-orbital so it cannot expand its octet, so it cannot have $10$ valence electrons, so the structure II is not acceptable. So, only statement A is correct.
Therefore, option (A) Anilinium ion has stable canonical structures I and III, is correct.
Note: Structure II is not acceptable. It does not represent the resonance structure, so we cannot draw it. We cannot say that II structure is no-aromatic or less stable because it is not an acceptable structure. As both structures I and II are the same hence both have the same stability. In resonance we always draw the acceptable structures with the same positions of atoms and completing their octet. The single structure representing the all resonating structure is known as resonance hybrid.
Complete step-by-step answer:
Resonance is the concept according to which a structure can be shown by more than one Lewis structure. All these structures are known as resonance structures or canonical structures.
The characteristics of resonance are as follows:
-The uncharged structure is more stable than the charged.
-Charge separation remains low. More electronegative atom bears negative charge and a more electropositive atom bears a positive charge.
-Equivalent resonating structures mean the delocalization of change at the same atoms, which are more stable resonating structures.
In structures I and III electrons are delocalized among all carbon atoms. Position of bonds and atoms are the same and the electrons are delocalized in form of double bond. Octet of each atom is complete. So, I and II are stable resonating structures.
In structure II, the electronic configuration of nitrogen is ${\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{3}}}$ . The valance electrons of nitrogen is $5$. It gets three electrons from three hydrogen atoms and two electrons from double bonds, so the total valence electrons of the nitrogen is $10$. Nitrogen does not have d-orbital so it cannot expand its octet, so it cannot have $10$ valence electrons, so the structure II is not acceptable. So, only statement A is correct.
Therefore, option (A) Anilinium ion has stable canonical structures I and III, is correct.
Note: Structure II is not acceptable. It does not represent the resonance structure, so we cannot draw it. We cannot say that II structure is no-aromatic or less stable because it is not an acceptable structure. As both structures I and II are the same hence both have the same stability. In resonance we always draw the acceptable structures with the same positions of atoms and completing their octet. The single structure representing the all resonating structure is known as resonance hybrid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




