
Carbon, silicon and germanium have four valence electrons each. These are characterised by valence and conduction bands separated by energy band gap respectively equal to ${\left( {{E_g}} \right)_C},{\text{ }}{\left( {{E_g}} \right)_{Si}}{\text{ and }}{\left( {{E_g}} \right)_{Ge}}$. Which of the following statements is true?
A. ${\left( {{E_g}} \right)_{Si}} < {\left( {{E_g}} \right)_{Ge}} < {\left( {{E_g}} \right)_C}$
B. ${\left( {{E_g}} \right)_C} < {\left( {{E_g}} \right)_{Ge}} < {\left( {{E_g}} \right)_{Si}}$
C. ${\left( {{E_g}} \right)_C} > {\left( {{E_g}} \right)_{Si}} > {\left( {{E_g}} \right)_{Ge}}$
D. ${\left( {{E_g}} \right)_C} = {\left( {{E_g}} \right)_{Si}} = {\left( {{E_g}} \right)_{Ge}}$
Answer
591.6k+ views
Hint: Here we will be comparing the energy gap of Si, C and Ge using band theory of solids. The separation between the bottom of the conduction band and top of the valence band is called the energy gap.
Complete step by step answer:
The band theory of solids is used to classify the materials into conductor, insulators and semiconductors. In this question, carbon belongs to insulator, Silicon and germanium belongs to semiconductor.
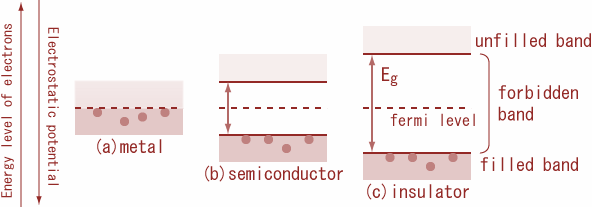
Conductors: the conductor is those substances which allow the electric current through them easily. It is because there is a large number of free electrons available in a conductor. The conductor's valence band and conduction band overlap with each other so that the energy gap as shown in the figure. Consequently, electrons are free to move, within the substance from the valence band to the conduction band. As a result, a very large number of electrons are available for conduction and such materials are called conductors. Therefore, even a small amount of potential difference is enough to develop an electric current.
Insulator: Insulators are those substances which do not allow the electric current to pass through it. In insulators, the valence band is full, while the conduction band is empty. The energy gap between the valence band and the conduction band is very large. Therefore, a high electric field is required to move an electron from the valence band to the conduction band.
Semiconductors: semiconductors are those substances whose electrical conductivity lies in between insulator and conductor. In a semiconductor, the valence band is almost filled and the conduction band is almost empty. The energy gap between the valence band and conduction is very small. At 0K, the valence band is completely filled and the conduction band is completely empty. Therefore, a semiconductor virtually behaves as an insulator at 0K. At room temperature, some electrons from the valence band cross over to the conduction band., giving rise to little conductivity to the semiconductors. As temperature increases, more valence electrons cross over to the conduction band, and conductivity increases.
We know that,
Energy gap of carbon is 5.4ev, silicon is 1.1ev and germanium is 0.7ev.
From this we can say that, ${\left( {{E_g}} \right)_C} > {\left( {{E_g}} \right)_{Si}} > {\left( {{E_g}} \right)_{Ge}}$
So, the correct answer is “Option C”.
Note:
In semiconductors, the energy gap value increases when the temperature decreases. Due to the amplitude of the various atomic vibrations, the interatomic spacings increase. This effect can be quantified by the expansion of the linear coefficient of the materials.
Complete step by step answer:
The band theory of solids is used to classify the materials into conductor, insulators and semiconductors. In this question, carbon belongs to insulator, Silicon and germanium belongs to semiconductor.

Conductors: the conductor is those substances which allow the electric current through them easily. It is because there is a large number of free electrons available in a conductor. The conductor's valence band and conduction band overlap with each other so that the energy gap as shown in the figure. Consequently, electrons are free to move, within the substance from the valence band to the conduction band. As a result, a very large number of electrons are available for conduction and such materials are called conductors. Therefore, even a small amount of potential difference is enough to develop an electric current.
Insulator: Insulators are those substances which do not allow the electric current to pass through it. In insulators, the valence band is full, while the conduction band is empty. The energy gap between the valence band and the conduction band is very large. Therefore, a high electric field is required to move an electron from the valence band to the conduction band.
Semiconductors: semiconductors are those substances whose electrical conductivity lies in between insulator and conductor. In a semiconductor, the valence band is almost filled and the conduction band is almost empty. The energy gap between the valence band and conduction is very small. At 0K, the valence band is completely filled and the conduction band is completely empty. Therefore, a semiconductor virtually behaves as an insulator at 0K. At room temperature, some electrons from the valence band cross over to the conduction band., giving rise to little conductivity to the semiconductors. As temperature increases, more valence electrons cross over to the conduction band, and conductivity increases.
We know that,
Energy gap of carbon is 5.4ev, silicon is 1.1ev and germanium is 0.7ev.
From this we can say that, ${\left( {{E_g}} \right)_C} > {\left( {{E_g}} \right)_{Si}} > {\left( {{E_g}} \right)_{Ge}}$
So, the correct answer is “Option C”.
Note:
In semiconductors, the energy gap value increases when the temperature decreases. Due to the amplitude of the various atomic vibrations, the interatomic spacings increase. This effect can be quantified by the expansion of the linear coefficient of the materials.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




