
Why is carboxylic acid weaker than HCl?
Answer
518.4k+ views
Hint: The acidity or basicity of a chemical is going to depend on the capability of donation of ${{H}^{+}}$ and $O{{H}^{-}}$ respectively. If the chemical is going to donate the ${{H}^{+}}$ ions very easily then the chemical is called strong acid and vice versa.
Complete answer:
- In the question it is asked why carboxylic acid is weaker than HCl.
- First, we should know about the dissociation of the acid in water.
- The dissociation of the carboxylic acid in water is as follows.
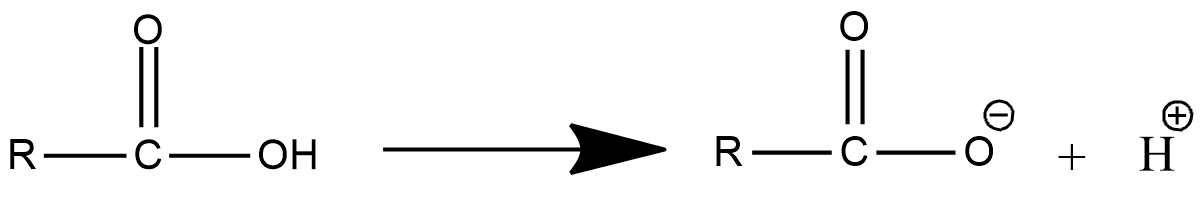
- In the above chemical dissociation of carboxylic acid, we can see that the carboxylic acid is going to convert into carboxylate anion and ${{H}^{+}}$ ion.
- But the carboxylate anion is going to exist in the following resonance structures.

- Due to the existence of the above chemical structures the ${{H}^{+}}$ ion formed at the time of dissociation is going to be grabbed by the formed resonance structures of the carboxylate anion.
- Coming to the dissociation of the HCl and it is as follows.
\[HCl\to {{H}^{+}}+C{{l}^{-}}\]
- The formed ${{H}^{+}}$ in the above chemical reaction is not going to be grabbed by the chloride ion which is formed in the above dissociation chemical reaction.
- Therefore, the carboxylate functional group is going to grab the ${{H}^{+}}$ ion which is formed after its dissociation but the HCl is not going to do like this.
- Therefore, the carboxylic acid is a weak acid when compared to HCl.
Note:
The strength of an acid is going to depend on the capability of the donation of the ${{H}^{+}}$ and not going to depend on the capability to accept the ${{H}^{+}}$ which is formed by itself during the dissociation in water.
Complete answer:
- In the question it is asked why carboxylic acid is weaker than HCl.
- First, we should know about the dissociation of the acid in water.
- The dissociation of the carboxylic acid in water is as follows.

- In the above chemical dissociation of carboxylic acid, we can see that the carboxylic acid is going to convert into carboxylate anion and ${{H}^{+}}$ ion.
- But the carboxylate anion is going to exist in the following resonance structures.

- Due to the existence of the above chemical structures the ${{H}^{+}}$ ion formed at the time of dissociation is going to be grabbed by the formed resonance structures of the carboxylate anion.
- Coming to the dissociation of the HCl and it is as follows.
\[HCl\to {{H}^{+}}+C{{l}^{-}}\]
- The formed ${{H}^{+}}$ in the above chemical reaction is not going to be grabbed by the chloride ion which is formed in the above dissociation chemical reaction.
- Therefore, the carboxylate functional group is going to grab the ${{H}^{+}}$ ion which is formed after its dissociation but the HCl is not going to do like this.
- Therefore, the carboxylic acid is a weak acid when compared to HCl.
Note:
The strength of an acid is going to depend on the capability of the donation of the ${{H}^{+}}$ and not going to depend on the capability to accept the ${{H}^{+}}$ which is formed by itself during the dissociation in water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




